Professor Eliezer J. Barreiro
This edition of the Annual Activities Report (AAR) from the National Institute of Science and Technology in Drugs and Medicines (INCT-INOFAR), referent to the year 2012, tries to describe and publicize all its activities that were carried out during the second year of the second decade of the new millennium. At this age, we live at the mercy of several and always new challenges, represented by countless variables, from climate phenomena that have not been fully understood and that bring us back to our social responsibility in the planet sustainability, to the management of diplomatic crises everywhere, which threaten the apparent world peace. It is not our place to rank these threats, but at the risk of being a little simplistic, we can admit that as frontiers for thought, all the challenges that we live with, whether to be aware of the reality and relevance of the global village we are members of, whether it is to understand, even if partially, the stage of economic, social, technological, or scientific that different nations are at. We must fully commit to ensure, preserve, and enhance the health of our populations. There is a strong and urgent need to understand, finally, that the population of the planet is its biggest asset. At a time when we have not yet overcome the social differences between and within nations, the issue of health care has become important and necessary enough so that we can enter the “Century of Life”.
Among many specialists, there is dialogue on the importance of health in the continuously improving quality of life we deserve, and in this subject, we must recognize that drugs, as active principles of medicines, used to prevent diseases and ensure health, are required tools. This way, knowing how to invent them, discover them, or create them, is consequently a theme that must be present in this future agenda.
In this context and with this understanding, INCT-INOFAR as a research network in drugs and medicines gathers scientists from different research institutes and Universities in Brazil, experts in some stage of the complex chain of innovation in drugs and medicines, acting in both dimensions of innovation in drugs: radical and incremental. This research network has been working jointly and achieving significant results in both innovation environments. A few myths were undone and overcome with the organization of the work of the several national institutions, distributed in eight states in Brazil, into a network, allowing for better management of the new knowledge created. Through systematic meetings, in person or otherwise, and continuous follow-up, we have ensured the necessary speed to the flow of information among the teams involved in the subprojects of INCT-INOFAR, so that we can meet the deadlines previously agreed upon for each of the projects. We have also achieved significant results for outreach actions concerning drug sciences, having edited booklets with content on their rational and safe use. Many of the practical results of these actions are published in the “Drugs Portal (Portal dos Fármacos)”, the spot for publicizing INCT-INOFAR.
Managed by a Scientific & Governance Advisory Committee (Comitê de Governança e Acompanhamento, CGA/INCT-NOFAR) made up of six specialists from different institutions and with interests in research areas related to those of the chain of innovation in drugs, INCT-INOFAR has a management model capable of identifying and ordering its priorities and defining its main goals with a pre-defined schedule of deadlines for activities, always renegotiated as needed for each subproject under study. Establishing hierarchy criteria for the classification, based on the current stage of each subproject in relation to the chain of innovation of drugs and medicines, we can establish the required dynamism desired for the actions of management and follow-up of the performance of each of the actors, optimizing them qualitatively.
Through a specific web portal, capable of assuring a notable standard of transparency to its actions, INCT-INOFAR has created a restricted access area, accessible with the use of passwords, which allows for the safe exchange of information among different research teams involved with the same current subproject. At the same time, an agile channel of communication between the Coordination and the researchers of the associated teams was created. Furthermore, when established by CGA/INCT-INOFAR, theme follow-up meetings were carried out, always with the presence of experts from outside the Institute as scientific consultants, to evaluate the progress obtained in certain areas where INCT-INOFAR acts, for comparison with other subprojects for reviewing priorities, if necessary. In May 2012, the VI Follow-Up and Evaluation Workshop by INCT-INOFAR took place, with the presence of Dr. Simon Campbell, a prestigious drug researcher, former research director for Pfizer in Sandwich, England, responsible for the discovery of several successful innovative drugs, as a consultant. His participation enabled us to enhance the vision of the development through the eyes of a pharmaceutical company that acts in radical innovation, balancing the management of the knowledge generated by the INCT-INOFAR team.
On this fourth edition of our Annual Activity Report, the activities that took place throughout 2012 are described, including public results of several research subprojects aimed at radical and incremental innovation. We have included quantitative data on the productivity of researchers connected to INCT-INOFAR and on the associated research and graduate programs, a list of our scholars at different levels and institutions, as well as our internationalization actions that made us present at international congresses. Initiatives capable of promoting the transfer of technology for the industrial sector in drugs and medicines, public or private, whether in radical or incremental innovation, were included in this AAR-2012.
Good reading.
Dr. Eliezer J. Barreiro
Scientific Coordinator INCT-INOFAR

INTRODUCTION
NATIONAL INSTITUTES OF SCIENCE AND TECHNOLOGY
In 2008, the Brazilian Government released the public announcement MCT/ CNPQ no014/2008, recruiting scientists to work in a network, in research areas strategic to the sustainable development of the country. This announcement has been so far the one with the greatest incentive to Science and Technology in Brazil.
At the time, some of the scientists associated with the Millenium Institute of Innovation and Development of Drugs and Medicines (IM-INOFAR) took on the challenge and presented a new project. So was born the “National Institute of Science and Technology of Drugs and Medicines” (INCT-INOFAR).
Like INCT-INOFAR, a total of 122 National Institutes of Science and Technology (INCTs) were created. Connecting groups of laboratories or associate research groups from different parts of the country, INCTs have a goal of acting in areas that are strategically important for the sovereignty of the country. INCT-INOFAR is in charge of health research aiming at the discovery of new drugs and medicines.
INCT OF DRUGS AND MEDICINES (INCT-INOFAR)
The National Institute of Science and Technology in Drugs and Medicines (INCT-INOFAR) is a research network that brings together renowned scientists from different research institutions and Universities in Brazil. Its mission is to act in the discovery of new drugs and medicines and in the search for new synthetic routes for generic drugs, as well as enhancing professional qualification at the undergraduate and graduate levels in Medicinal Chemistry and Pharmacology, the core disciplines involved in pharmaceutical discovery.
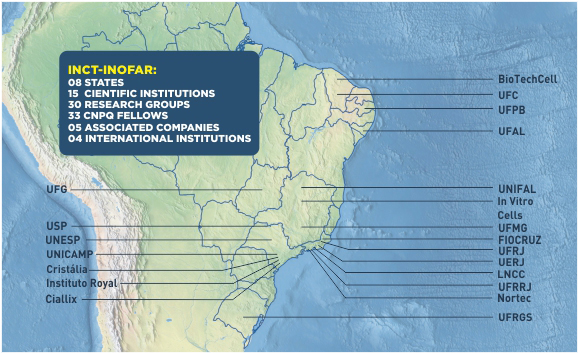
Made up of nearly one hundred scientists from 30 research groups focused on (radical) pharmaceutical innovation and (incremental) generic drug research, INCT-INOFAR is present in 15 teaching and research institutions in 8 different Brazilian states.
With the task of qualifying new human resources capable of acting in important stages ofthe process of discovery/invention of new drugs – from the election of the therapeutic-target to the conclusion of bioassays at the pre-clinical stage - INCT-INOFAR contributes to identify and reduce important deficiencies in the chain of pharmaceutical innovation.
Parallel to laboratory research, INCT-INOFAR acts in society, increasing awareness of the Science it practices, encouraging the correct and responsible use of medicines. It maintains the Drugs Portal, a website created to publicize pharmaceutical science. In compliance with the original edict, INCT-INOFAR carries out health education actions aimed at children, among other activities that inform the population on the rational use of drugs.





MISSION
- To organize national scientific competences into an effective and productive network of pharmaceutical and medicines research;
- To support scientific research subprojects aimed at the chain of innovation in drugs and medicines;
- To act in the incremental innovation of drugs through generic drugs;
- To study and develop total synthetic routes for current and future generic drugs, as well as advanced intermediates and raw materials that are strategic to the sector;
- To contribute to the qualification and education of personnel in Medicinal Chemistry & Pharmacology;
- To promote scientific awareness of drugs and medicines, and therefore contribute effectively for their rational and safe use.
PHARMACEUTICAL INNOVATION
With the contribution of its entire research network, INCT-INOFAR studies and develops several subprojects in radical innovation and also acts in incremental innovation, studying new total synthesis routes for generic drugs.
In the field of radical innovation, the Institute aims at the discovery/invention of original substances, active in in vivo pharmacological models, widely validated, capable of originating new pharmaceutical candidates in different therapeutic classes. The research areas of interest for INCT-INOFAR are: inflammation, pulmonary diseases, pain, central nervous system, cardiovascular system, and chemotherapy for cancer and for so-called neglected diseases, in particular leishmaniasis.
In the area of incremental innovation, INCT-INOFAR leads projects that are focused on the search for new synthetic routes, efficient and accessible, for generic drugs that are already in the market – and that are important tools in health public policy and in the pharmaceutical care provided to the population – as well as for those drugs that are about to have their patents expire, which represent new business perspectives for the pharmaceutical business sector.
INCT-INOFAR RESEARCH AREAS
- Inflammation;
- Pulmonary Diseases;
- Pain;
- Diabetes;
- Central Nervous System;
- Cardiovascular System;
- Chemotherapy: antineoplastic and leishmanicide;
- Generic Drugs.
GENERIC DRUGS
In spite of the advances due to the 13 years since the creation of the Generics Law (no 9.787/1999) in Brazil, sadly, most national pharmaceutical companies still simply formulate and package active principles imported from distant markets like China, India, and Korea.
Working at trying to reverse this “Indian Route” process, INCT-INOFAR has made efforts in the study and development of new total synthesis routes for generic medicines, with the goal of transferring the technology acquired to local pharmaceutical industries.
By studying and developing total synthesis routes for generic drugs, advanced intermediates and strategic raw materials for the sector, INCT-INOFAR research makes way for the production of active principles at reduced prices in Brazil.
Ever since it was created in 2009, INCT-INOFAR has developed new synthetic routes for atorvastatin, sunitinib, and fluoxetine.
Atorvastatina
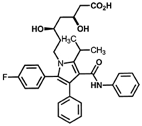
At the same month when the patent for Lipitor™/ Pfizer expired in Brazil (December 2010), INCT-INOFAR researchers announced the discovery of a new synthesis route for the production of its active principle, atorvastatin. A continuous use drug to reduce cholesterol that is widely used, Lipitor™ was the best-selling pharmaceutical in the world during its history, reaching US$ 150 billion in sales during its patent (1991-2011).
Those responsible for the research, which had great repercussion in both local and foreign press throughout 2011, were Prof. Luiz Carlos Dias and Dr. Adriano Siqueira Vieira from the Institute of Chemistry of the State University of Campinas (Unicamp), the latter as an INCT-INOFAR scholar. The synthesis route for atorvastatin has been patented and it represents an important technological asset to INCT-INOFAR. Since then, INCT-INOFAR has been negotiating the production of this generic with Brazilian pharmaceutical companies.
Sunitinib
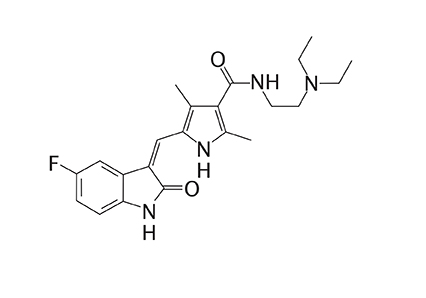
Recommended to fight certain types of stomach cancer, sunitinib is the active principle of Sutent®/ Pfizer, a high cost medication – around R$ 11,000 per box with 28 pills – which, unfortunately, is not yet made available through the Public Health Care System (SUS) and that, due to that, is the source of many lawsuits, since it represents the primary indication in those cases.
The sunitinib synthesis route was finished in September 2011 by Prof. Angelo da Cunha Pinto and by Dr. Barbara Vasconcellos da Silva, from the Institute of Chemistry of the Federal University of Rio de Janeiro (UFRJ). With the discovery of the new synthesis route for sunitinib, Brazil can beprepared to produce the medication, reducing its production cost when the patent for the drug expires in the country, or in other circunstances, as defined by the Brazilian government.
Fluoxetine
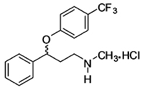
Antidepressant medication in the class of selective serotonin reuptake inhibitors, fluoxetine was marketed by Eli Lilly Laboratories under the name of Prozac™, until the patent for the medication expired in August 2001, allowing the production of generic versions. Fluoxetine is part of the National List of Essential Medicines (RENAME) and is available through the Popular Drugstore Program due to the technological knowledge of its synthesis, and it is an important achievement by INCT-INOFAR.
Considered the controlled substance with the largest demand in the public health network, most of the fluoxetine consumed in Brazil is imported. Due to the social and market impact of this medication in the country, INCT-INOFAR has made efforts towards the discovery of a new synthesis route for generic fluoxetine. So far, the group led by Prof. Luiz Carlos Dias from Unicamp has prepared 2g of the active principle, using a new and efficient methodology, so that the pharmaceutical can be prepared in larger quantities, in a faster, more practical, cheaper manner, with less environmental impact.
MULTIDISCIPLINARY RESEARCH NETWORK
The process of pharmaceutical innovation has clear inter- and multidisciplinary characteristics that demand competences in distinct areas that make up the health sciences.
INCT-INOFAR articulates academic scientific research groups in different areas into a network, covering the stages of the process of invention of new drugs, which go from the election of the therapeutic target to the conclusion of bioassays in the pre-clinical trial stage, using quantitative and qualitative analytical methods, as well as clinical pharmacology.
The INCT-INOFAR research team is made up of specialists in different subjects such as medicinal chemistry, pharmacology, organic chemistry, toxicology, organic synthesis, biochemistry, computational chemistry, structural biology, spectroscopy, chemistry of natural products, among other related areas.
SCIENTIFIC EXCHANGE
INCT-INOFAR is present in 15 research and teaching institutions, in 8 different Brazilian states, cooperating actively to reduce the regional scientific imbalance in Brazil, as well as contributing to strengthen the expertise in a sector that is strategic to the country.
By enabling researchers in different institutions in several geographical areas to associate their work, INCT-INOFAR promotes exchange between the larger research centers and the emerging research groups.
Cooperative work is a way for INCT-INOFAR to contribute to the increase of the scientific and technological output in emerging research groups, especially in the Mid-West and Northeast regions, benefitting the qualification at undergraduate and graduate levels. Throughout these three years since INCT-INOFAR was created, the benefits for the advancement of these emerging groups in scientific terms have been notable.
INCT-INOFAR RESEARCH GROUPS: Laboratories and seniors scientists
RIO DE JANEIRO
1.FIOCRUZ
Inflammation Laboratory
Marco Aurelio Martins CV-Lattes
National Public Health School
Francisco Jose Roma Paumgartten CV-Lattes
2.UERJ
Department of Pharmacology
Theresa Christina Barja-Fidalgo CV-Lattes
3.UFRJ
Laboratory of Evaluation and Synthesis of Bioactive Substances – LASSBio
Carlos Alberto Manssour Fraga CV-Lattes
Lidia Moreira Lima CV-Lattes
System of Information on the Chemical Industry – SIQUIM
Adelaide Maria de Souza Antunes CV-Lattes
Laboratory of Pulmonary Investigation
Patricia Rieken Macedo Rocco CV-Lattes
Laboratory of Biochemical and Molecular Pharmacology
Francois Germain Noel CV-Lattes
Laboratory of Cardiovascular Pharmacology
Gisele Zapata Sudo CV-Lattes
Laboratory of Muscular Excitement-Contraction Coupling
Roberto Takashi Sudo CV-Lattes
Laboratory of Molecular Virology I
Jose Nelson dos Santos Silva Couceiro CV-Lattes
Laboratory of Natural Products and Chemical Transformations
Angelo da Cunha Pinto CV-Lattes
Laboratory of Support to Technological Development
Francisco Radler de Aquino Neto CV-Lattes
Laboratory of Pharmacology of Inflammation and Nitric Oxide
Patricia Dias Fernandes CV-Lattes
4.UFRRJ
Institute of Exact Sciences
Carlos Mauricio Rabello de Sant’Anna CV-Lattes
5.LNCC
Group of Molecular Modeling of Biological Systems
Laurent Emmanuel Dardenne CV-Lattes
SÃO PAULO
6.USP-RP
Laboratory of Pain and Inflammation
Fernando de Queiroz Cunha CV-Lattes
7.UNESP ARARAQUARA
Nucleus of Bioassays, Biosynthesis, and Ecophysiology of Natural Products (NUBBe)
Vanderlan da Silva Bolzani CV-Lattes
8.UNICAMP
Laboratory of Synthetic Organic Chemistry
Luiz Carlos Dias CV-Lattes
MINAS GERAIS
9.UFMG
Group of Innovation in Organic and Inorganic Compounds with Pharmacological Activity
Heloisa de Oliveira Beraldo CV-Lattes
10.UNIFAL
Laboratory of Phytochemistry and Medicinal Chemistry
Claudio Viegas Junior CV-Lattes
Marcia Paranho Veloso CV-Lattes
RIO GRANDE DO SUL
11.UFRGS
GENOTOX-ROYAL Unit
Joao Antonio Pegas Henriques CV-Lattes
Laboratory of Experimental Psychopharmacology
Stela Maris Kuze Rates CV-Lattes
GOIAS
12.UFG
Laboratory of Bioconversion
Valeria de Oliveira CV-Lattes
Rosangela de Oliveira Alves Carvalho (contributor) CV-Lattes
Laboratory of Pharmacology and Cellular Toxicology (partner)
Marize Campos Valadares Bozinis CV-Lattes
Laboratory of Medicinal Pharmaceutical Chemistry
Ricardo Menegatti CV-Lattes
Laboratory of Cardiovascular Pharmacology (partner)
Matheus Lavorenti Rocha CV-Lattes
ALAGOAS
13.UFAL
Laboratory of Pharmacology and Immunity
Magna Suzana Alexandre Moreira CV-Lattes
CEARA
14.UFC
Unit of Clinical Pharmacology
Manoel Odorico de Moraes CV-Lattes
Laboratory of Pharmacology of Inflammation and Cancer
Ronaldo de Albuquerque Ribeiro CV-Lattes
PARAÍBA
15.UFPB
Laboratory of Toxicological Assays (LABETOX)
Margareth de Fatima Formiga Melo Diniz CV-Lattes
QUALIFICATION OF HUMAN RESOURCES
So that a truly innovative drug is discovered, we must have diverse and extremely qualified personnel to carry out successfully all the stages in the chain of innovation.
Collaborating to perfect Brazilian expertise in the discovery/invention of new drugs and medicines, INCT-INOFAR strongly acts in the qualification of human resources in the different research centers it is associated with.
At INCT-INOFAR, scientific training is enhanced at all academic levels: undergraduate, graduate, doctoral, and post-doctoral. As part of this training, graduate students connected to the studied subprojects are encouraged to take part in the scientific exchange between participating laboratories with specific expertise, as to make the agreed upon goals happen within deadlines that meet the project needs.
Through the scientific exchange promoted and encouraged by INCT-INOFAR, the Institute contributes not only to the high education of new researchers, but also to keep senior researchers updated. Maintaining professionals that are renowned for their talent in the country is also one of the premises for INCT-INOFAR.
Premises
- Qualification of human resources;
- Scientific-academic Exchange;
- Updating of senior researches;
- Maintenance of renowned researches in the country.
INCT-INOFAR researchers actively take part in education and qualification of human resources, through their connections to 16 Graduate Programs of renowned academic merit throughout Brazil.
Over half of the Graduate Programs with the participation of INCT-INOFAR are ranked 6 or 7 (out of 7).
GRADUATE PROGRAMS WITH INCT-INOFAR RESEARCHES
(USP/RP) Graduate Program in BIOLOGICAL SCIENCES (PHARMACOLOGY) M / D - CAPES - 7
http://www.radioribeirao.ccrp.usp.br/pos_graduacao.asp
(UNICAMP) Graduate Program in CHEMISTRY – M / D - CAPES 7
http://www.iqm.unicamp.br/posgraduacao/
(UFRJ) Graduate Program in CHEMISTRY M / D – CAPES 7
(UNESP/ARAR) Graduate Program in CHEMISTRY M / D- CAPES 6
http://fi.com.br/projetos/unesp/
(UERJ) Graduate Program in BIOSCIENCES - CAPES 6
http://www.pgbiologia.uerj.br/
(UFC) Graduate Program in PHARMACOLOGY - CAPES 6
http://www.fisfar.ufc.br/posgrad/
(FIOCRUZ) Graduate Program in CELULAR AND MOLECULAR BIOLOGY M / D – CAPES 6
http://www.fiocruz.br/iocensino/cgi/cgilua.exe/sys/start.htm?sid=6
(UFMG) Graduate Program in CHEMISTRY – M/D – CAPES 6
(UFRGS) Graduate Program in PHARMACEUTICAL SCIENCES M / D - CAPES 6
(UFPB) Graduate Program in BIOACTIVE NATURAL AND SYNTHETIC PRODUCTS – M / D – CAPES 5
https://sites.google.com/a/ltf.ufpb.br/pgpnsb/
(UFRJ) Graduate Program in PHARMACOLOGY AND MEDICINAL CHEMISTRY M / D – CAPES 4
http://www.farmaco.ufrj.br/posgraduacao/index.html
(UNIFAL) Graduate Program in CHEMISTRY M – CAPES 4
http://www.unifal-mg.edu.br/ppgquimica/
(UFRRJ) Graduate Program in CHEMISTRY M / D - CAPES 4
http://www.ice.ufrrj.br/posgrad/
(UFAL) Graduate Program in HEALTH SCIENCES M – CAPES 3
http://www.ufal.edu.br/unidadeacademica/icbs/pos-graduacao/ciencias-da-saude
(UNIFAL) Graduate Program in PHARMACEUTICAL SCIENCES M – CAPES 3
http://www.unifal-mg.edu.br/ppgcienciasfarma/
(UFG) Graduate Program in PHARMACEUTICAL SCIENCES M - CAPES 3
http://mestrado.farmacia.ufg.br/pages/23204
Source: Triennial Evaluation Report 2010 – 2007 to 2009, CAPES.
See the full list of master and doctoral theses advised by INCT-INOFAR researchers and finished in 2012 at page 168 (pdf).
CAPES THESES AWARD - CHEMISTRY
Every year, the Coordination for the Improvement of Higher Education Personnel (Capes) gives out an award for the best doctoral theses in different areas of knowledge, the Capes Theses Awards. In 2012, INCT-INOFAR won the Award for Chemistry.
Thesis “Synthesis of ferrocenyl oxyndoles and the study of isatin chloration with trichloroisocyanuric acid”,
Author: Barbara Vasconcellos da Silva – CV-Lattes
Advisor: Prof. Angelo da Cunha Pinto
Institution: Institute of Chemistry at UFRJ
Aside from the Capes Theses Award in Chemistry, the research above was also awarded a prize from Paulo Gontijo Institute. (http://www.ipg.org.br/home.php).


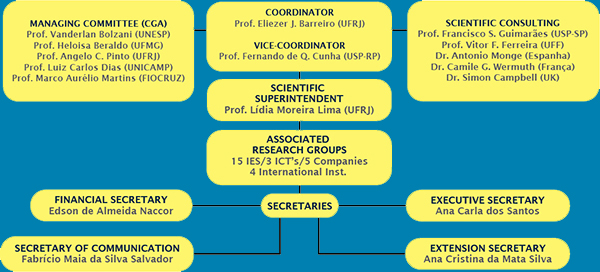
ORGANIZATIONAL STRUCTURE OF INCT-INOFAR
The organizational structure of INCT-INOFAR is made up of a Coordinator, a Vice-Coordinator, and the Scientific Advisory and Follow-Up Committee (Comitê de Governança e Acompanhamento /CGA). This committee is a deliberative and consulting collegiate, which acts on the strategic planning of INCT-INOFAR activities.
The Scientific Superintendence supports the Coordination, acting in the technical-scientific evaluation of projects under study, also acting on following up on previously agreed upon deadlines.
INCT-INOFAR has the participation, on a confidential basis, of specialist external consultant that provide scientific support in the evaluation of the projects under study, aiming at the optimization of its research activities. In a few projects, the consultants suggest also occasional route changes needed to fulfill the essential objective of the Institute, which is to contribute to the discovery of new national pharmaceuticals.
The network of scientific competences of INCT-INOFAR is made up of 30 research groups, located in 15 institutions, in 8 different Brazilian states. Each research group associated to INCT-INOFAR is led by a specialist, responsible for the scientific interaction of its team among itself and among the other Institute teams.
The Financial, Executive, and Media Affairs Secretaries provide the necessary support to the full development of the research and publicizing activities carried out by INCT-INOFAR and they are physically located in the Health Sciences Center at UFRJ, the administrative headquarters of the Institute.
With a goal of expanding its outreach actions in Pharmaceutical Sciences, on April 2012, INCT-INOFAR created the Extension Secretary. Acting alongside the other Secretaries, the Extension Secretary has the challenge of spreading the Health Education projects done by INCT-INOFAR, bringing the discussion on the correct use of medicines to public schools.
ASSOCIATED COMPANIES
INCT-INOFAR has the support, though yet informal, of pharmaceutical companies and related companies such as Cristalia Chemical Pharmaceutical Products Ltd., Royal Institute, In Vitro Cells Technological Research S.A. and Ciallyx Laboratories & Consulting.
In Vitro Cells (Pesquisa Toxicológica)
In Vitro Cells – Toxicological Research S.A. is a technology based company located at Biominas Foundation (Belo Horizonte, MG). Its founders are professors at the Federal University of Minas Gerais (UFMG) in the areas of Toxicology and Biochemistry. The company is an INCT-INOFAR partner to carry out in vitrobioassays to evaluate the safety and efficacy of new drug candidates developed by the Institute.
Cristalia Chemical Pharmaceutical Products (Cristália Produtos Químicos Farmacêuticos)
Cristalia Chemical Pharmaceutical Products Ltd. is a pharmaceutical company associated with INCT-INOFAR, capable of supporting the carrying out, with an onus, the possible stages of pharmacotechnical development of new compound-prototypes that reach this advanced stage of the chain of innovation in drugs and medicines. Under terms of non-disclosure and confidentiality, Cristalia will benefit, if it is interested in doing so, of the information on the projects under study, by expressing an interest in the established deadlines in internalizing the technologies developed at INCT-INOFAR. For technological transfer to happen, the Innovation Agency at UFRJ, and its equivalent from another INCT-INOFAR research institution connected to the specific project will directly negotiate with the interested parties, including financial backers.
Royal Institute (Instituto Royal)
Toxicology is a very delicate stage that may absolve or condemn forever a drug candidate compound. INCT-INOFAR prioritizes cyto-, muta-, and genotoxicity studies, as well as acute toxicology, with molecules that have displayed attractive pharmacological activity, as early as possible in the chain of pharmaceutical innovation. To ensure that all the pre-clinical toxicology stages are accredited in good laboratory practices (GLP), INCT-INOFAR has partnered with the Royal Institute, which is the result of the merge of two toxicology laboratories located in different universities. Genotox-Royal Institute, located at UFRGS, carries out the genetic toxicity studies, while Unitox-Royal, located at the University of Santo Amaro (Unisa – SP) is responsible for animal toxicity tests.
Ciallyx Laboratories & Consulting (Ciallyx Laboratórios & Consultoria)
Ciallyx Laboratories & Consulting is a company located at CIETEC (Incubating Center for Technological Companies) that carries out efficacy studies (proof of concept) and safety studies (toxicological studies and assays) for new molecules, medicines, and formulations. Ciallyx gets results by following national and international protocols under strict quality standards using as a guide the international guidelines of Good Laboratory Practices – GLP. The company is an INCT-INOFAR partner to conduct in vivo bioassays for the evaluation of safety and efficacy of new drug candidates developed by the Institute.
BiotechCell (Toxicologia Aplicada, Desenvolvimento de Produtos e Projetos)
The BiotechCell® is an enterprising biotechnology company in the Northeast of Brazil, rising in the scientific community through an ideal of researchers who sought to combine their extensive academic experience to the management of technological innovation and services. We operate in the market providing services on “in vivo” and “in vitro” antitumor tests, toxicology, preclinical applied toxicogenetics and human toxicogenetics biomonitoring. Our staff is highly trained to provide fast and accurate solutions to attend your needs.
As part of our working philosophy, the BiotechCell® has a well-established Quality System on which all our procedures, studies and analysis are based on. Thereby, on this principle, the company has strived to follow the evolution of the market, as well as performing non-clinical studies required by regulatory agencies for the registration of pesticide products, their components and related products, pharmaceuticals, cosmetics, wood preservatives, food additives and feed, veterinary products, cleaning products, industrial chemicals, genetically modified organisms, remediation, among others, aiming to evaluate the environmental risk and the risks for human health.
INTERNATIONAL AGREEMENTS

INCT-INOFAR has directed efforts at internationalizing its research network, through the signing of international cooperation agreements. This internationalization is done through recommendations from the National Council for Scientific and Technological Development (CNPq) and adopts the philosophies of the Science without Borders program.
The goal is to give international visibility to Science, Technology, and Innovation activities in Brazil. From this internationalization, new cooperation networks can be established, and these networks can create training opportunities for students, at both the undergraduate and graduate level, abroad.
![]() GERMANY
GERMANY
As far as the agreement that the Federal University of Rio de Janeiro (UFRJ) established with the University of Aveiro, Portugal, INCT-INOFAR, in September 2012, established a specific agreement to develop research jointly with the Department of Chemistry from the same university. The covenant was signed in loco by Prof. Jose A. F. Cavalheiro of the University of Aveiro and by INCT-INOFAR coordinator, Prof. Eliezer J. Barreiro.
On January 2012, Prof Jose Cavalheiro was in Rio de Janeiro to take part in the XVIII Summer School in Medicinal Pharmaceutical Chemistry. The event, supported by INCT-INOFAR researchers, has the goal of, during a week of academic summer break, gathering students from different parts of the country to discuss recent topics in Medicinal Pharmaceutical Chemistry with researchers that are experts on the subject.

 ITALY
ITALY
INCT-INOFAR also has a covenant with the research group led by Prof. Pier G. Baraldi of the University of Ferrara, Italy. This closeness brought Prof. Baraldi to Brazil three times (2004, 2009 and 2012) to be part of the Summer School in Medicinal Pharmaceutical Chemistry and has made it possible for a doctoral exchange to take place at the University of Ferrara. The scientific exchange of INCT-INOFAR researcher Rodolfo do Couto Maia, of the Graduate Program in Chemistry (PGQu-UFRJ), took place between February and July 2011, and was supervised by Prof. Pier Baraldi, in Italy, and the advisement of Professors Carlos Alberto M. Fraga and Eliezer J. Barreiro, in Brazil.
 URUGUAY
URUGUAY
The CAPES-UDELAR Program has the goal of promoting, through joint research projects, the exchange of professors and researchers from Brazil and from Uruguay, in several fields of knowledge. As part of this Program, INCT-INOFAR keeps a covenant with researchers from the Department of Organic Chemistry of the Faculty of Chemistry from the Universidad Nacional de La Republica (UdelaR). The research projects led by Professors Hugo Cerecetto and Mercedes Gonzalez of Udelar are related to the design, the synthesis, and the pharmacological evaluation of potential drugs.
A key part of the research covenant is the planning, synthesis, and determination of pharmacological properties of new N-acylhydrazone (NAH) derivates with important in vitro trypanocide activity. This class of bioactive substances, which has been the object of continued research efforts by LASSBio – UFRJ, is being synthesized in the Brazilian laboratory in a quantity large enough to allow its complexation by several metals. The formation of the metallic compound and the evaluation of its antiparasite properties in vitro are being done by the Uruguayan team.
OTHER INTERNATIONALIZATION ACTIONS
Parallel to its international agreements, INCT-INOFAR makes efforts towards specific cooperation between its researchers and renowned foreign researchers.
Under confidentiality, INCT-INOFAR has the participation of international consultants that provide scientific help in the evaluation of projects under study. Prof. Antonio Monge, director of the department of pharmaceutical chemistry of the University of Navarra, Spain, and Dr. Camille G. Wermuth, founder of Prestwick Chemical and retired professor of the Faculty of Pharmacy of the Louis Pasteur University in France, are part of the permanent team of INCT-INOFAR consultants.
With the goal of bringing the view of the pharmaceutical industry to the evaluation of its research projects, in 2012, INCT-INOFAR asked renowned scientist Dr. Simon Campbell to be its international consultant. Responsible for the discovery of Viagra™ (sildenafil) as well as for other important medicines for Pfizer pharmaceutical industries, Simon Campbell came to Brazil to be part of the VI INCT-INOFAR Evaluation and Follow-Up Meeting. (Read more about it on page 97).
INCT-INOFAR SUBPROJECTS IN STUDY
Incremental Innovation
01. Synthesis of sunitinib
Prof. Eliezer J. Barreiro (UFRJ) CV Lattes
Prof. Angelo da Cunha Pinto (UFRJ) CV Lattes
Prof. Barbara Vasconcelos (UFRJ) CV Lattes
02. Synthesis of fluoxetine
Prof. Eliezer J. Barreiro (UFRJ) CV Lattes
Prof. Luiz Carlos Dias (UNICAMP) CV Lattes
Dr. Adriano V. Siqueira (UNICAMP) CV Lattes
03. Synthesis of atorvastatin
Prof. Eliezer J. Barreiro (UFRJ) CV Lattes
Prof. Luiz Carlos Dias (UNICAMP) CV Lattes
Dr. Adriano V. Siqueira (UNICAMP) CV Lattes
Radical Innovation
04. Evaluation of leishmanicide activity of a series of semicarbazone and hydrazine-N-acylhydrazone derivates
Prof. Magna Suzana Alexandre Moreira (UFAL) CV-Lattes
05. New 5-aril-furfuril-N-acylhydrazone functionalized derivates with power anti-inflammatory and analgesic action: LASSBio-1609 and LASSBio-1636
Prof. Carlos Alberto Manssour Fraga (UFRJ) CV-Lattes
06. Discovery of new antitumor pharmaceutical candidates analog to combrestatin A4
Prof. Lidia Moreira Lima (UFRJ) CV-Lattes
07. Development of new antiasthmatic pharmaceutical prototypes (LASSBio-596)
Prof. Patricia Rieken Macedo Rocco (UFRJ) CV-Lattes
Prof. Lidia Moreira Lima (UFRJ) CV-Lattes
08. Study of N-phenylpiperazine derivates functionalized as prototypes for the development of new atypical antipsychotics
Prof. Stela Maris Kuze Rates (UFRS) CV-Lattes
Prof. Carlos Alberto Manssour Fraga (UFRJ) CV-Lattes
09. Study of the potential anti-inflammatory effect of LASSBio 897 compound, in silicosis and asthma models
Prof. Patricia Machado Rodrigues e Silva (FIOCRUZ – RJ) CV-Lattes
Prof. Marco Aurelio Martins (FIOCRUZ – RJ) CV-Lattes
10. Semicarbazone benzaldehyde (BS)
Prof. Heloisa de Oliveira Beraldo (UFMG) CV-Lattes
11. Development of anti-arthritic pharmaceutical candidates, MAPK p-38 modulators
Prof. Lidia Moreira Lima (UFRJ) -CV-Lattes
12. Therapeutic potential of new vasodilator (LASSBio 1289) in arterial and pulmonary hypertension
Prof. Gisele Zapata Sudo (UFRJ) CV-Lattes
13. Pharmacological evaluation of new neuroactive Zolpidem derivates
Prof. Roberto Takashi Sudo (UFRJ) CV-Lattes
14. Planning, synthesis, structural characterization and pharmacological evaluation of new candidates to anti-inflammatory and neuroactive drugs
Prof. Claudio Viegas Junior (UNIFAL) CV-Lattes
15. Pharmacological and toxicological evaluation of new drugs for the prevention and treatment of myocardiopathy and neuropathy caused by diabetes mellitus
Prof. Gisele Zapata Sudo (UFRJ) CV-Lattes
16. Development of new anti-inflammatory and analgesic compound candidates from safrole
Prof. Lidia Moreira Lima (UFRJ) CV-Lattes
17. Impact of nanoparticle therapy with the thymuline gene in a chronic allergic asthma model
Prof. Patricia Rieken Macedo Rocco (UFRJ) CV-Lattes
18. Study for the identification of new sulfonamide compounds effective in the control of pulmonary inflammation caused by silica in mice
Prof. Patricia Machado Rodrigues e Silva Martins (FIOCRUZ-RJ) CV-Lattes
19. Technological prospecting of new generics in Brazil
Prof. Adelaide Maria de Souza Antunes (UFRJ) CV-Lattes
20. Prospecting of opportunities in new generics and innovative generics
Prof. Adelaide Maria de Souza Antunes (UFRJ) CV-Lattes
21. Planning of structural changes aiming the optimizing of affinity of the selective inhibitor of IKK2 enzyme, LASSBio-1524
Prof. Laurent Emmanuel Dardenne (LNCC) CV-Lattes
22. Theoretical investigation of the action mechanism of dialkylphosphoril hydrazones as 5-phosphate isomerase ribose enzyme of Trypanosoma cruzi and plasmodium falciparum
Prof. Carlos Mauricio R. de Sant’Anna (UFRRJ) CV-Lattes
23. Implementation and validation of pre-clinical model for the evaluation of teratogenic effect of bioactive substances: evaluation of LASSBio 468 and LASSBio 596 prototypes
Prof. Aloa Machado de Souza (UFRJ) CV-Lattes
24. “In silico” prediction and “in vitro” production of bioconversion of human metabolites candidates to pharmaceutical prototypes
Prof. Valeria de Oliveira (UFG) CV-Lattes
25. Planning, synthesis, and pharmacological evaluation of vectorized neuroactive and self-organized drugs
Prof. Ricardo Menegatti (UFG) CV-Lattes
26. Evaluation of the antitumor activity of new molecules structurally planned from the imatinib prototype
Prof. Patricia Dias Fernandes (UFRJ) CV-Lattes

DESIGN, SYNTHESIS, AND PHARMACOLOGICAL EVALUATION OF N-ACYLHYDRAZONES AND NOVEL CONFORMATIONALLY CONSTRAINED COMPOUNDS AS SELECTIVE AND POTENT ORALLY ACTIVE PHOSPHODIESTERASE-4 INHIBITORS.
Arthur E. Kümmerle; Martine Schmitt; Suzana V. S. Cardozo; Claire Lugnier; Pascal Villa; Alexandra B. Lopes; Nelilma C. Romeiro; Helene Justiniano; Marco A. Martins; Carlos A. M. Fraga; Jean-Jacques Bourguignon and Eliezer J. Barreiro. Journal of Medicinal Chemistry 55 (2012) 7525-7545. DOI: 10.1021/jm300514y
Type 4 phosphodiesterase (PDE) are the major cyclic AMP metabolizing isoenzymes found in inflammatory cells1,2. A number of studies have emphasized the therapeutic potential of PDE4 inhibitors for controlling inflammatory airway disorders, such as asthma and chronic obstructive pulmonary disease (COPD)3,4. In the current study, we described a novel pharmacological profile for N-acylhydrazone (NAH) derivatives. When this important chemotype was flanked by two substituted aromatic ring systems, Ar1 and Ar2, a new series of PDE inhibitors was generated. Specific substituents directed the selectivity toward different PDE isoforms. It was demonstrated that the N-methylation and substitution at Ar2 was critical, particularly when methoxy groups were located at both meta and para positions. These changes clearly favored the formation of compounds with selective submicromolar inhibitory activity upon the enzyme PDE4. On the other hand, substitutions at Ar1 increased the potency towards PDE4, amplified the selectivity profile towards other isoenzymes, or completely re-oriented the selectivity profile to other PDE subtypes. The SAR analysis highlighted the most promising compounds, which confirmed anti-TNF-α properties both in vitro and in vivo. In another set of experiments, based on the conformational analysis of N-methyl-NAH and thanks to the 3D modeling approach, describing the docking of a prototypical PDE4 inhibitor, zardaverine5, in the active site of PDE4, novel heterocyclic NAH-mimetic compounds were designed, synthesized and tested in vitro. Interestingly, the quinazoline derivative (19) appeared as a conformationally restricted NAH mimetic and showed similar PDE4-inhibitory and anti-TNF-α properties compared with the corresponding free rotating NAH derivative 8a. In addition, the most interesting NAH derivatives were tested orally and found effective in inhibiting the LPS-induced airway hyper-reactivity and lung inflammation, emphasizing the therapeutic potential of this novel class of biologically active compounds.
Our working hypothesis was very effective, since it allowed the identification of a valuable hit (8a) originated from a versatile chemical library (NAHs), followed by rational design of a novel class of heterocyclic NAH-mimetic (19) with remarkable in vitro and in vivo activity. These compounds should be further investigated as molecular prototypes in drug discovery for asthma and COPD.
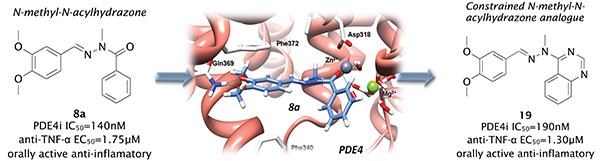
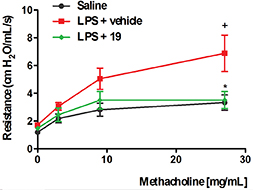
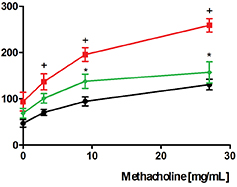
Helping to breath. The inflammatory response can lead to several lung diseases, including chronic obstructive pulmonary disease (COPD) and severe asthma, both with increasing death rate worldwide. Such diseases are difficult to treat with the steroidal anti-inflammatory drugs (the best anti-inflammatory agents available so far) probably because these drugs favor the survival of the leukocyte polymornuclear neutrophils. Another important reason for this refractoriness is that IL-17, which accounts for the neutrophil recruitment into the inflammatory focus, is resistant to glucocorticoids. The study of Kümmerle and collaborators published in the Journal of Medicinal Chemistry, in 2012, identified new N-methyl-N-acylhydrazone (NAH) derivatives as selective PDE4 inhibitors. These compounds presented selective sub-micromolar activity on PDE4 and anti-anti-TNF-α properties in vitro and in vivo. Furthermore, as administered by the oral route, these compounds inhibited lung neutrophil accumulation and airway hyper-reactivity triggered by LPS in mice. Notably, differently from what was observed with rolipram and other PDE4 inhibitors, the NAHs did not show evidence of pro-emetic profile. These findings strongly suggest that the class of N-methyl-N-acylhydrazones shows indeed much promise as an alternative for anti-inflammatory therapy for COPD and asthma.
DISCOVERY OF NOVEL ORALLY ACTIVE ANTI-INFLAMMATORY N-PHENYLPYRAZOLYL-N-GLYCINYL-HYDRAZONE DERIVATIVES THAT INHIBIT TNF - α PRODUCTION.
Lacerda, R, B.; Silva, L. L.; Lima, C. K. F.; Miguez, E.; Miranda, A. L. P.; Laufer, S. A.; Barreiro, E. J.; Fraga, C. A. M. PLoS ONE 7 (2012) e46925. DOI: 10.1371/journal.pone.0046925
The production of proinflammatory cytokines, e.g., TNF-a, IL-1β and IL-6, is a key factor in chronic inflammatory diseases, such as rheumatoid arthritis [1]. Due to the role of cytokines in various inflammatory diseases, many pharmaceutical companies have made efforts to develop new orally active substances that can modulate the production of proinflammatory cytokines. Tumor necrosis factor-alpha (TNF-α) is a pleiotropic cytokine that possesses proinflammatory and osmoregulator actions [2]. It is the major cytokine mediator of acute inflammation, it activates platelets, and it is also involved in the genesis of fever and anemia. The currently available anti-TNF-α strategies involve either administration of anti-TNF-α antibodies or soluble TNF receptors to remove circulating TNF-α [3]. Despite the approval of anti-TNF-α drugs, the appearance of side effects resulting from the debilitating actions of these drugs on the immune system highlights the necessity of identifying new alternative mechanisms to modulate the actions of pro-inflammatory cytokines [4,5]. One of the most promising targets involved in modulating the production of pro-inflammatory cytokines is the mitogen-activated protein kinase (MAPK) pathway, particularly p38 MAPK, a serine–threonine protein kinase that has been identified as a molecular target of the pyridinyl-imidazole derivatives. Over the years, a large number of structurally diverse p38α and p38β MAPK inhibitors have been developed with both enhanced potency and specificity. Most of the p38 MAPK inhibitors are ATP competitors [6], but a new class of allosteric inhibitors has also been reported [7]. For example, BIRB-796 [8] (3) produces a conformational reorganization of the kinase that prevents ATP binding and activation.
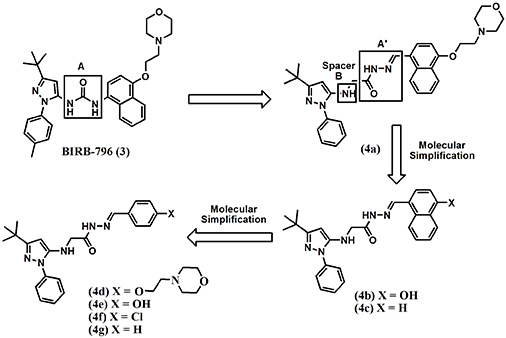
In this context, the present work describes the synthesis of novel N-phenylpyrazolyl-N-glycinyl-hydrazone derivatives 4a-g, which were designed as structural analogues of the p38 MAP kinase inhibitor BIRB-796 (3), and the investigation of their anti-cytokine and anti-inflammatory properties. For the proposed derivatives (4a-g), we investigated the replacement of the urea subunit of BIRB-796 (3) by a N-acylhydrazone unit [9] (A’, Figure 1), which was attached to the N-phenyl-pyrazole nucleus through an NHCH2 spacer (B, Figure 1).
Furthermore, we performed a series of molecular simplifications in the functionalized naphthyl framework attached to the imine unit of the NAH group of compound 4a to better understand the structure-activity relationships (Figure 1).
All of the novel synthesized compounds described in this study were evaluated for their in vitro capacity to inhibit tumor necrosis factor a (TNF-a production in cultured macrophages and in vitro MAPK p38a inhibition. The two most active anti-TNF-α derivatives (Figure 2), LASSBio-1504 (4a) and LASSBio-1506 (4f), were evaluated to determine their in vivo anti-hyperalgesic profiles in carrageenan-induced thermal hypernociception model in rats. Both compounds showed anti-inflammatory and antinociceptive properties comparable to SB-203580 used as a standard drug, by oral route at a dose of 100 µmol/kg (Figure 3). This bioprofile is correlated with the ability of NAH derivatives (4a) and (4f) suppressing TNF-a levels in vivo by 57.3 and 55.8%, respectively.
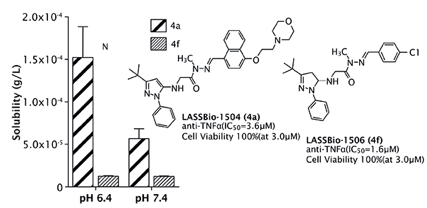
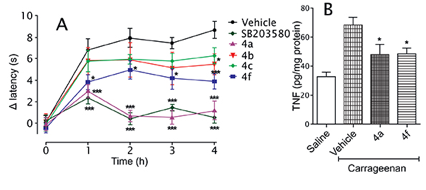
Moreover, we also evaluated the in vitro metabolic stability of derivatives LASSBio-1504 (4a) and LASSBio-1506 (4f) when placed in contact with preparations of liver and plasma of rats. The two N-acylhydrazone derivatives were resistant to oxidative microsomal metabolism, but the derivative 4a was about four times more resistant than derived 4f to plasma degradation. Taken together, these results indicate that the plasma stability associated to the better aqueous solubility are responsible for the better in vivo pharmacological profile shown by the NAH derivative LASSBio-1504 when given orally.
References:
[1] Kapoor M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J. P.; Fahmi, H. Nat. Rev. Rheumatol. 2011, 7, 33.
[2] Locksley, R. M.; Killeen, N.; Lenardo, M. J. Cell 2001, 104, 487.
[3] Lin, J.; Ziring, D.; Desai, S.; Kim, S.; Wong, M.; Korin, Y. Clin. Immunol. 2008, 126, 13.
[4] Palladino, M. A.; Bahjat, F. R.; Theodorakis, E. A.; Moldawer, L. L. Nat. Rev. Drug Discovery 2008, 2, 736.
[5] Bongartz, T.; Sutton, A. J.; Sweeting, M. J.; Buchan, I.; Matteson, E. L. JAMA 2006, 295, 2482.
[6] Frantz, B.; Klatt, T.; Pang, M.; Parsons, J.; Rolando, A.; Williams H. Biochemistry 1998, 37, 13846.
[7] Pargellis, C.; Tong, L.; Churchill, L.; Cirillo, P. F.; Gilmore, T. Nature Struct. Biol. 2002, 9, 268.
[8] Regan, J.; Breitfelder, S.; Cirillo, P.; Gilmore, T.; Graham, A. G. J. Med. Chem. 2002, 45, 2994.
[9] Duarte, C. D.; Barreiro, E. J.; Fraga, C. A. M. Mini-Rev. Med. Chem. 2007, 7, 1108.
COMMENTS FROM AUTHOR
After the structural planning, the target NAH compounds were synthesized from a multi-step route, starting from cheap materials and applying classical synthetic methodologies, evaluated in vitro as TNF-alpha inhibitors and p38 MAPK inhibitors, and then, evaluated as anti-inflammatory agents with potential to treat cronic inflammatory diseases. Despite being structurally designed from a p38 MAP kinase inhibitor these compounds displayed their pharmacological profile through another mechanism of action, i.e. the ability to promote an expressive reduction of TNFa levels in vitro and in vivo, which is a good indicative of possible differences during the clinical trials in comparison of classical p38 inhibitors. The evaluation of some pharmacokinetic properties as aqueous solubility, lipophilicity and metabolic stability confirmed the potential for oral administration of these NAH derivatives, as evidenced by their expressive anti-inflammatory and anti-hyperalgesic activity after oral administration in rats. Moreover, their effects in mBSA antigen induced arthritis model in mice indicated that these compounds could be useful to treat cronic inflammatory diseases, where few and expensive therapeutical options are available. For example, the biotech anti-TNF derivatives, infliximab, adalimumab, etc… For this reason, the N-acylhydrazone prototypes LASSBio-1504 and LASSBio-1506 represent new orally active low weight drug candidates able to block TNF-α, as a low cost interesting alternative to the biotech compounds, that need to be used through parenteral route and presented a lot of side effects, as the induced-immunossupression.
DOCKING, SYNTHESIS AND ANTI-DIABETIC ACTIVITY OF NOVEL SULFONYLHYDRAZONE DERIVATIVES DESIGNED AS PPAR-GAMMA AGONISTS
Gisele Zapata-Sudo, Lídia M. Lima, Sharlene L. Pereira, Margarete M. Trachez, Filipe P. da Costa, Beatriz J. Souza, Carlos E. S. Monteiro, Nelilma C. Romeiro, Éverton D. D’Andréa, Roberto T. Sudo and Eliezer J. Barreiro. Current Topics Med. Chem. 12 (2012), 2037-2048. DOI: 10.2174/1568026611212190002
Diabetes is a metabolic disorder characterized by hyperglycemia. When not properly controlled, complications include neuropathy, coronary artery disease, and renal failure. This work describes the virtual screening and synthesis of a novel series of sulfonylhydrazone derivatives designed as peroxisome proliferator-activated receptor gamma (PPARγ) agonists and investigation of the analogs for hypoglycemic activity in a murine model of diabetes.
After the selection of LASSBio-331(5) as the ligand with the best theoretical affinity for the target PPARγ (Fig. 1), alterations in its acidic subunity were proposed to build a new series of compounds (Chart 1).
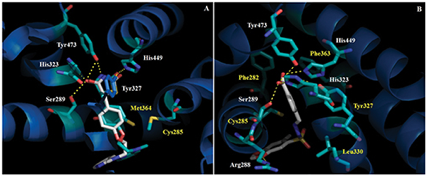
Figure 1 - The structures of PPARγ ligand binding domain in complex with 1 (S-rosiglitazone, A) and 5 (LASSBio-331, E-isomer, B) (stick representations) obtained by flexible docking. Hydrogen bonds in dashed yellow lines. White legends= hydrogen bonding residues; Yellow legends= putative hydrophobic interactions. Co-crystallized rosiglitazone in blue carbon atoms. Docked ligands in gray carbon atoms.
This new series was planned using nonclassical bioisosterism [1] between carboxylic acid (5), tretrazole (16) and suphonic acid (17) functional groups; and exploring an isomerism strategy to design the regioisomers 14 and 15 (Chart 1).
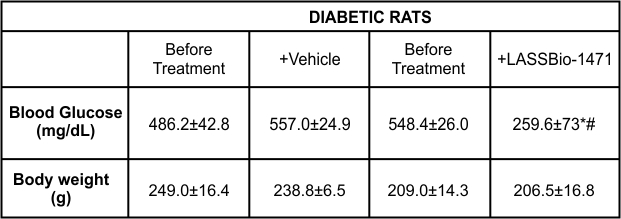
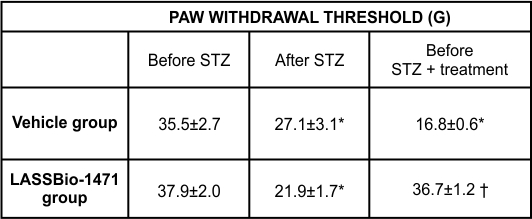
Male Wistar rats received an intravenous injection of streptozotocin (STZ) (60 mg/kg) to induce diabetes. A significant increase in blood glucose concentration was observed 4 weeks after diabetes induction compared to the glucose levels of nondiabetic animals. Daily intraperitoneal administration of LASSBio-1471 for 7 days produced a significant reduction in blood glucose levels compared to vehicle-treated diabetic rats, indicating the hypoglycemic activity of this compound. (Table 1).
Table 1 - Evaluation of blood glucose levels and body weight in diabetic rats treated with vehicle (DMSO) or LASSBio-1471 (20 mg/kg, i.p.) for 7 days.
Four weeks after STZ injection, we evaluated the mechanical allodynia of diabetic rats after a single intraperitoneal injection of LASSBio-1471 or vehicle (DMSO). Paw withdrawal threshold was significantly reduced in diabetic rats compared to the nondiabetic group. This parameter was partially recovered in rats treated with LASSBio-1471. At 30 minutes after LASSBio-1471 administration, the paw withdrawal threshold increased from 21.9 ± 1.7 (before injection) to 34.3 ± 1.5 g (P < 0.05) (Fig. 2).
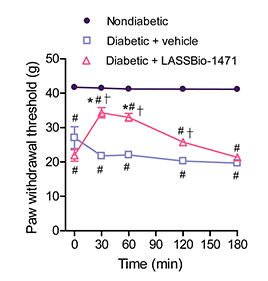
Mechanical allodynia was also observed in diabetic rats treated with vehicle (DMSO) or LASSBio-1471 (20 mg/kg, i.p.) for 7 days. The paw withdrawal threshold was 27.1 ± 3.1 g before treatment and 16.8 ± 0.6 g at 7 days after the beginning of treatment. The paw withdrawal threshold of LASSBio-1471-treated diabetic rats was 21.9 ± 1.7 g before treatment and 36.7 ± 1.2 g after 7 days of treatment (P < 0.05; Table 2).
Table 2 - Evaluation of mechanical allodynia in diabetic rats treated with vehicle (DMSO) or LASSBio-1471 (20 mg/kg, i.p.) for 7 days.
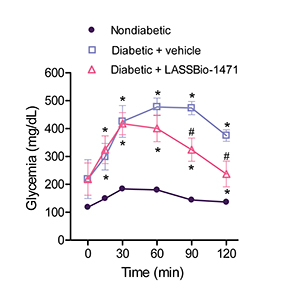
Long-term administration of LASSBio-1471 for 7 days in diabetic rats resulted in a significant improvement in oral glucose tolerance following oral glucose loading. Rats treated with LASSBio-1471 had a significant reduction in glucose levels to 237.1 ± 46.1 mg/dL (Fig. 3) after 120 minutes of oral glucose administration. These results indicated the anti-hyperglycemic activity of the sulfonylhydrazone derivative.
In this study, LASSBio-1471 exhibited hypoglycemic activity in rats with STZ-induced diabetes, as indicated by reduced blood glucose levels after prolonged treatment. Additionally, LASSBio-1471 exhibited analgesic effects, as demonstrated by improvement in mechanical allodynia in STZ-induced neuropathy. PPARγagonists such as LASSBio-1471 may have beneficial effects on hyperglycemic rats. Thiazolidinediones are pharmacological PPARγ agonists that increase insulin sensitivity, reduce blood glucose and circulating free fatty acid levels, and inhibit inflammatory pathways [2-5]. Our results indicated that LASSBio-1471 had a hypoglycemic effect in STZ-injected rats by activating PPARγ suggested by molecular docking studies.
The novel PPARγ agonist LASSBio-1471 is a promising substance, with beneficial effects on reducing hyperglycemia and diabetic neuropathy.
References:
[1] Lima, L.M.; Barreiro, E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem., 2005, 12, 23-49.
[2] Willson, T,M.; Cobb, J.E.; Cowan, D.J.; Wiethe, R.W.; Correa, I.D.; Prakash, S.R.; Beck, K.D.; Moore, L.B.; Kliewer, S.A.; Lehmann, J.M. The structure–activity relationship between peroxisome proliferator-activated receptor gamma agonist and the antihyperglycemic activity of thiazolidinediones. J. Med. Chem., 1996, 39, 665-668.
[3] Mudaliar, S.; Henry, R.R. New oral therapies for type 2 diabetes mellitus: The glitazones or insulin sensitizers. Annu. Rev. Med., 2001, 52, 239-257.
[4] Yki-Jarvinen, H. Thiazolidinediones. N. Engl. J. Med., 2004, 351, 1106-1118.
[5] Evans, J.L.; Lin, J.J.; Goldfine, I.D. Novel approach to treat insulin resistance, type 2 diabetes, and the metabolic syndrome: Simultaneous activation of PPARalpha, PPARgamma, and PPARdelta.Curr. Diabetes Rev., 2005, 1, 299-307.
N4-PHENYL-SUBSTITUTED 2-ACETYLPYRIDINE THIOSEMICARBAZONES: CYTOTOXICITY AGAINST HUMAN TUMOR CELLS, STRUCTURE-ACTIVITY RELATIONSHIP STUDIES AND INVESTIGATION ON THE MECHANISM OF ACTION.
Soares, M.A.; Lessa, J.A.; Mendes, I.C.; Da Silva, J.; Santos, R.G.; Salum, L.B.; Daghestani, H.; Andricopulo, A. D.; Day, B.W.; Vogt ,A.; Pesquero, J. L.; Rocha, W.; Beraldo, H. Bioorg. Med. Chem. 20 (2012) 3396-3409. DOI: 10.1016/j.bmc.2012.04.027
In 2030, an estimated 12 million deaths from cancer are estimated [1]. Breast cancer affects more than one million women every year. The high mortality is related to resistance of breast tumor cells to current therapy [2]. Malignant gliomas are lethal cancers originating in the central nervous system. They are very common in pediatric patients. The most aggressive, astrocytoma, is referred to as glioblastoma multiforme [3]. The low tolerance of the central nervous system to conventional chemotherapeutic agents impairs the effectiveness of the treatment. Hence, it is important to search for novel drug candidates.
Thiosemicarbazones are a class of compounds with wide range of pharmacological applications [4]. a(N)-heterocyclic thiosemicarbazones have been extensively investigated as potential anticancer agents [5]. This search led to the onset of clinical studies of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP; Triapine®) [6]. The antitumor activity of α(N)-heterocyclic thiosemicarbazones has been related to their ability to inhibit ribonucleoside diphosphate reductase (RDR), a rate-limiting enzyme in DNA syntheses that catalyses the conversion of ribonucleotides into deoxiribonucleotides [5,6].
The cytotoxic activity of thiosemicarbazones against a variety of human solid tumor cell lines as well as leukemic cells has been reported by other authors [7a] and by our group. We demonstrated that N4-phenyl 2-acetylpyridine thiosemicarbazone (H2Ac4Ph) and its N4-ortho-, -meta- and -para-chlorophenyl and N4-ortho-, -meta- and -para-tolyl derivatives show cytotoxicity at nanomolar concentrations against malignant glioma [7b].
The development of new anticancer drug candidates requires the evaluation of the possible modes of action involved in the process of cancer cell death. Apoptotic as well as non-apoptotic mechanisms have been identified. Autophagy is characterized by an increase in the number of autophagosomes, vesicles that surround cellular organelles. Subsequently, autophagosomes merge with lysosomes and digest the organelles, leading to cell death. Apoptosis and autophagy are predominantly distinct. However, cross-talk between them has been demonstrated. Several chemotherapeutic agents, hormonal therapies, natural compounds, cytokines, gene therapies, microtubule disturbing agents and radiotherapy have shown to trigger autophagic cell death in a panel of cancer cells [8].
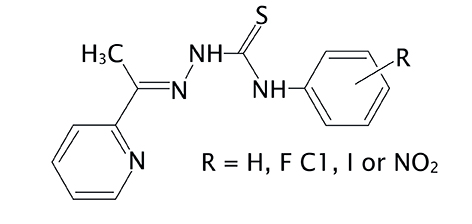
We now evaluated the cytotoxicities of N4-phenyl 2-acetylpyridine thiosemicarbazone (H2Ac4Ph) (1) and its N4-ortho-, -meta- and -para-fluorophenyl- (H2Ac4oFPh, H2Ac4mFPh, H2Ac4pFPh) (2-4), N4-ortho-, -meta- and -para-chlorophenyl- (H2Ac4oClPh, H2Ac4mClPh, H2Ac4pClPh) (5-7), N4-ortho-, -meta- and -para-iodophenyl- (H2Ac4oIPh, H2Ac4mIPh, H2Ac4pIPh) (8-10) and N4-ortho-, -meta- and -para-nitrophenyl- (H2Ac4oNO2Ph, H2Ac4mNO2Ph, H2Ac4pNO2Ph) (11-13) derivatives (Fig 1) against MCF-7 (breast adenocarcinoma), U87 (glioblastoma multiforme expressing wild-type p53 protein) and T98G (glioblastoma multiforme expressing mutant p53) human malignant tumor cells. A preliminary analysis of the compounds’ mode of action was carried out. The effects of the thiosemicarbazones on tubulin assembly as well as on cellular microtubule organization and mitotic arrest were also investigated.
In the structure-activity relationship (SAR) studies properties of interest were molecular surface area, theoretical octanol–water partition coefficients (log P), dipole moment, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies, which were correlated to pIC50 (−log IC50). Molecular surface area may offer information on stereo features required for drug–receptor interactions. Log P and dipole values may give some insights on the degree of lipophilicity of the molecules. HOMO and LUMO energies are related to ionization potential and electron affinity, respectively. These frontier orbitals are associated to the molecule’s reactivity. HOMO energy is closely related to susceptibility to electrophilic attack while LUMO energy is related to susceptibility to nucleophilic attack.
1H NMR spectra of the thiosemicarbazones indicated a mixture of the E (95–87%) and Z (13–5%) configurational isomers. Crystal structure determinations showed that the thiosemicarbazones adopt the EE conformation in relation to the C7–N2 and N3–C8 bonds.
SAR data for the E isomers indicated similar correlations between the chemical descriptors and cytotoxicity against MCF-7 and U87 cells. Different correlations were found between descriptors and cytotoxicity against T98G and U87 cells. The former is wild-type while the latter is a p53-mutant cell. Hence the mechanisms of thiosemicarbazones’ cytotoxic effect may be different in these two cell lineages. Correlations were observed between the cytotoxic activities against MCF-7 and U87 cells and the molecular surface area (R = −0.71 and −0.60 for MCF-7 and U87 cells, respectively), the HOMO energy (R = 0.69 and 0.67 for MCF-7 and U87 cells, respectively) and the charge on sulfur (R =0.71 and 0.68 for MCF-7 and U87 cells, respectively). Thus, the smaller the molecular surface area, the higher the cytotoxic activity against MCF-7 and U87 cells. The direct correlation observed between the HOMO energies and the activities against MCF-7 and U87 cells is an interesting result since the HOMO energy is related to the molecules’ reactivity. In addition, the direct correlation between cytotoxicity and the charge on sulfur indicates that the more negatively charged the sulfur atom, the higher anti-proliferative effect against MCF-7 and U87 cells. HOMO energy and negative charge on sulfur are correlated (R = 0.90) and both properties are related to the activities against MCF-7 and U87 cells. Thus, the sulfur atom may play an important role in the thiosemicarbazones’ reactivity and therefore, in their cytotoxic activity. Comparison of HOMO density plots for all E isomers revealed distinct electronic delocalization among the studied thiosemicarbazones, which may partially account for the differences in activity of these compounds, although other parameters may not be excluded. Regarding correlation between the properties of E isomers and activity against T98G cells, only an inverse correlation was observed between cytotoxicity and molecular surface area (R = −0.60). Correlations observed for E isomers were also found for the Z isomers. In addition, for the Z isomers cytotoxicity against T98G cells is correlated to the LUMO energy (R = 0.63) and the dipole (R = 0.62).
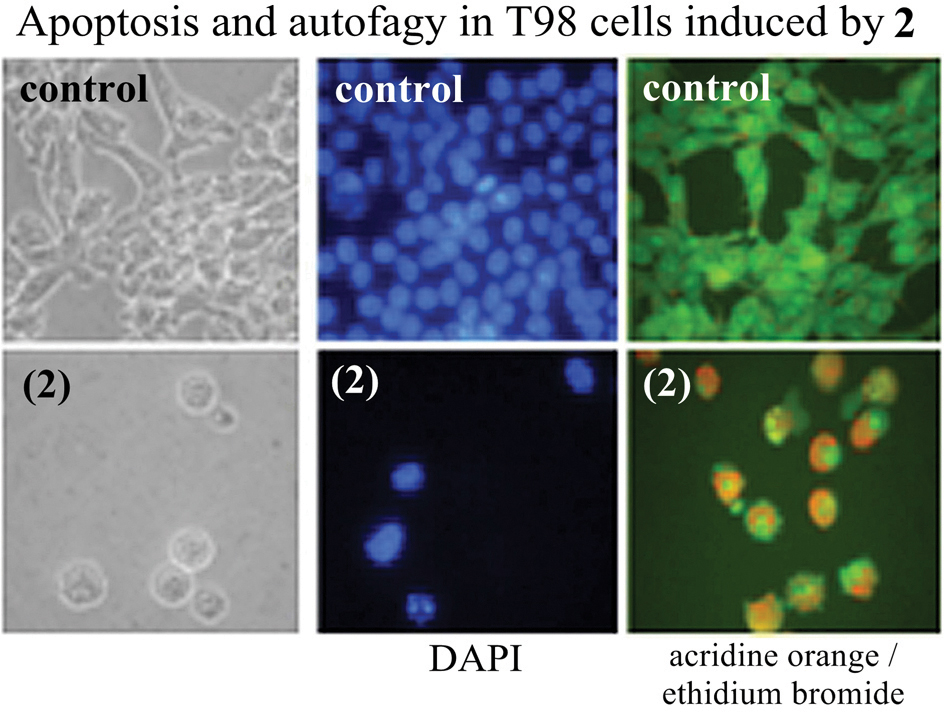
Treatment with 1–13 induced membrane and nuclear alterations characteristics of programmed cell death on U87, T98G and MCF-7 cells. Morphological changes such as irregularities in cellular shape, cell shrinkage and membrane blebbing were observed. Chromatin condensation and DNA fragmentation were also noticed in all treated cells when stained with 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI) (Fig. 2). Hence, apoptosis induction would be at least in part responsible for the reduction of cell survival.
Acridine orange/ethidium bromide (AO/EB) staining can be used to differentiate live, apoptotic and necrotic cells. Under AO/EB staining control cells showed cytoplasm and nucleus with homogeneous green with minimal orange fluorescence indicative of healthy cells. Treated cells presented chromatin condensation (bright green fragments) and absence of EB fluorescence, indicating preserved membrane. These features are typical of early apoptosis. Moreover, treated cells presented large acidic compartments in the cytoplasm, and visible red fluorescence, indicating the presence of autophagolysosomes, characteristic of cells engaged in autophagy. Our results suggest that the studied thiosemicarbazones were able to induce two types of programmed cell death.
References:
[1] WHO—World Health Organization”, http://www.who.int/cancer.
[2] (a) Coughlin, S.S.; Ekwueme, D.U.; Cancer Epidemiol., 2009, 33, 315.
[3] Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P.; Acta Neuropathol., 2007, 114, 97.
[4] Beraldo, H.; Gambino, D.; Mini-Rev. Med. Chem., 2004, 4, 31.
[5] Finch, R.A.; Liu, M.; Grill, S.P.;. Rose, W.C.; Loomis, R.; Vasquez, K.M.; Cheng, Y.; Sartorelli. A.C.; Biochem. Pharmacol., 2000, 59, 983.
[6] Gojo, I.; Tidwell, M.L.; Greer, J.; Takebe, N.; Seiter, K.; Pochron, M.F.; Johnson, B.; Sznol, M.; Karp, J.E.; Leuk. Res., 2007, 31, 1165.
[7] (a) Quiroga, A.G.; Navarro-Ranninger C.; Coord. Chem. Rev., 2004, 248, 119. (b) Lessa, J.A.; Mendes, I.C.; Da Silva, P.R.O.; Soares, M.A.; Dos Santos, R.G.; Speziali, N.L.; Romeiro, N.C.; Barreiro, E.J.; Beraldo. H.; Eur. J. Med. Chem., 2010, 45, 5671 and references therein.
[8] Kondo, Y.; Kondo, S.; Autophagy, 2006, 2, 85.
COMMENTS FROM AUTHOR
In previous works we had demonstrated that 2-acerylpyridine-derived thiosemicarbazones are cytotoxic to glioma cells. We now showed that a family of N4-phenyl-substituted 2-acerylpyridine thiosemicarbazones exhibit cytotoxicity against MCF-7 (breast cancer), and U87 and T98G (glioma) tumor cells at nanomolar doses. The mechanism of antitumor activity of thiosemicarbazones involves inhibition of ribonucleoside diphosphate reductase (RDR), an enzyme that catalyses the conversion of ribonucleotides into deoxiribonucleotides during DNA biosyntheses. However these compounds are believed to act on multiple targets. The present investigation on the modes of action of the studied thiosemicarbazones revealed that they were able to induce both apoptosis and autophagy in the tested tumor cell lineages. Despite their abilities to inhibit tubulin assembly at high doses and to provoke cellular microtubule disorganization, the compounds did not behave as mitotic arresters. We believe that the present work constitutes a contribution to the understanding of the complex modes of cytotoxic action of thiosemicarbazones. In addition, the high cytotoxic effects of the compounds, together with their good therapeutic indexes, suggest that they might constitute attractive antitumor drug candidates.
INVESTIGATION OF TRYPANOTHIONE REDUCTASE INHIBITORY ACTIVITY BY 1,3,4-THIADIAZOLIUM-2-AMINIDE DERIVATIVES AND MOLECULAR DOCKING STUDIES.
Raquel F. Rodrigues*, Denise Castro-Pinto, Aurea Echevarria, Camilla M. dos Reis, Catarina N. Del Cistia, Carlos Mauricio R. Sant’Anna, Filipa Teixeira, Helena Castro, Marilene Canto-Cavalheiro, Leonor L. Leon, Ana Tomás. Bioorganic & Medicinal Chemistry 20 (2012) 1760–1766. DOI:10.1016/j.bmc.2012.01.009
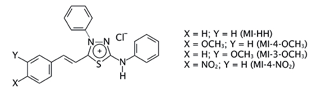
Parasitic protozoa of the family trypanosomatidae are the causative agents of many significant tropical diseases, including African trypanosomiasis, Chagas’ disease, and Leishmaniasis. Trypanosoma cruzi is a protozoan parasite from the order Kinetoplastida that causes Chagas’ disease. Previous studies from our group have shown that mesoionic derivatives of the 1,3,4-thiadiazolium-2-aminide class inhibit the in vitro growth of Leishmania amazonensis, L. braziliensis, L. chagasi and T. cruzi. In the present study, we sought to elucidate the target of mesoionic derivatives on Leishmania sp. and T. cruzi. Three species of Leishmania were selected to this work, L. (L) amazonensis, L. (V) braziliensis, and L. (L) infantum. The enzyme trypanothione reductase (TryR) is a validated drug target in trypanosomatids, as it was shown to be essential for the survival of these parasites by protecting them against oxidative stress. This enzyme is dependent of NADPH andcatalyzes the reduction of trypanothione disulphide [T(S)2] dithiol to trypanothione. Here, the effects of mesoionic derivatives (Fig. 1) on TryR from parasite extracts and on recombinant enzymes from L. infantum and T. cruzi were evaluated.
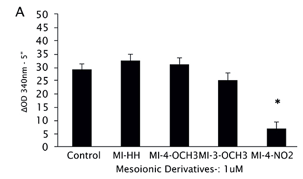
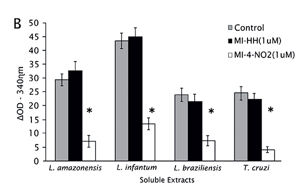
The effect of the derivatives was evaluated in soluble extracts from late log phase of L. amazonensis promastigotes (Fig. 2A). The reaction was followed by the NADPH consumption; the control was considered the highest consumption as 100% TryR activity. Only MI-4-NO2 was able to modify NADPH consumption (76 % enzyme inhibition), so the same assay was carried out with the soluble extracts from the other parasites using MI-4-NO2 and MI-HH, as a reference (Fig. 2B).
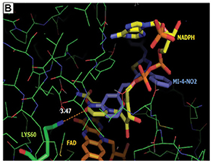
From the results obtained in TryR activity assays in soluble extracts of parasites, NADPH consumption assays were carried out using recombinant enzymes from L. infantum (LiTryR) and T. cruzi (TcTryR). It was demonstrated that a pre-incubation with 1µM of MI-4-NO2 inhibited 76% LiTryR and 69% TcTryR (p<0.005) activities. Besides, the addition of 1µM of MI-HH did not alter NADPH consumption in comparison with the control. The analysis of enzyme kinectic of LiTryR was carried out in order to confirm the mode of action of MI-4-NO2 and MI-HH. Only MI-4-NO2 was observed to lower values of maximum reaction rate with little or no apparent effect on the Michaelis constant, which are characteristics effects of a noncompetitive inhibitior.
A molecular docking study with the TryR of the four parasite species was implemented with the GOLD software (CCDC). For the docking into L. infantum and T. cruzi TryR, the crystal structures available in PDB were used; for L. amazonensis and L. braziliensis TryR, it was necessary the previous construction of comparative 3D models with the Swiss Model server. As TryR is a FAD-dependent oxydoreductase, which utilizes NADPH as an electron donor. The four mesoionic compounds were predicted to effectively dock into the substrate and the FAD binding sites. The docking of the compounds into the NADPH site, however, was predicted as better than into the FAD and substrate binding sites. It was observed that the MI-4-NO2 binds differently than the other mesoionic compounds (Fig. 3A) into this site; the nitro group of MI-4-NO2 makes a H bond with Lys60 side chain. The planar p-nitro-phenyl group makes p-p interactions with the isoalloxazine ring of the FAD cofactor, which is responsible for its redox action, so it is expected that these interactions could interfere with the enzyme activity (Fig. 3B).
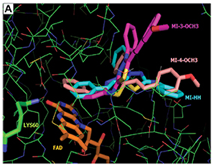
Relevance of the work: In the present work, a probable molecular mechanism of action, TryR inhibition, was identified for the nitro derivative of a series of active mesoionic compounds against Leishmania sp. and T. cruzi parasites, based on enzyme inhibition and kinetic data, and theoretical results. Other mechanisms of action, independent of TryR inhibition, remain to be established for the remaining compounds of the active series. It is possible that more than one metabolic pathway in the parasites is involved. The results obtained in this work will be useful for future studies of mesoionic compounds as part of a drug discovery program against Leishmaniasis or Chagas’ disease.
TOLL-LIKE RECEPTOR 9 ACTIVATION IN NEUTROPHILS IMPAIRS CHEMOTAXISAND REDUCES SEPSIS OUTCOME.
Silvia C. Trevelin, José C. Alves Filho, Fabiane Sônego, Walter Turato, Daniele C. Nascimento, Fabricio O. Souto, Thiago M. Cunha, Ricardo T. Gazzinelli, Fernando Q. Cunha. Critical Care Medicine 40 (2012) 2631-7. DOI: 10.1097/CCM.0b013e318258fb70.
Sepsis is the main cause of death in critically ill patients that occurs when the host reaction to infection becomes inadequate, resulting in bacteremia and a systemic inflammatory response [1,2]. In spite of neutrophils be important players in the control of microorganisms; during severe sepsis they fail in migrate to the site of infection, what was extensively correlated with poor outcome in animal sepsis models [3]. Moreover, neutrophils from septic patients have impaired response to CXCL8, chemokine recognized by CXCR2, an important receptor leading neutrophil recruitment [4].
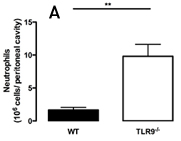
The mechanisms that govern the failure of neutrophil recruitment are complex, involving the activation of toll-like receptors (TLRs) 2 and 4 [5,6,7], nitric oxide [8], TNF-alpha [9] and heme-oxygenase products [10]. Adding more information, we reported in Critical Care Medicine that TLR9 deficiency enhances neutrophil migration toward the focus of infection in mouse model of severe cecal ligation and puncture (S-CLP)-induced sepsis (Figure 1a). TLR9 is an intracellular receptor that recognizes unmethylated cytosine-phosphate-guanine (CpG) motifs present in microbial DNA and mitochondrial DNA.
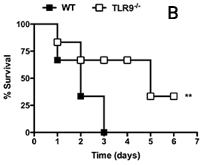
The enhanced neutrophil migration to peritoneal cavity observed in TLR9 deficient mice with S-CLP was associated with higher bacterial clearance, lower neutrophil sequestration in lungs and levels of TNF-alpha and CXCL2 in serum than their wild type (WT) counterparts. Differently of 100% of mortality observed in WT, approximately 40% of TLR9 deficient mice survived after given S-CLP within 7 days of observation (Figure 1b).
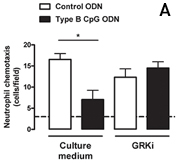
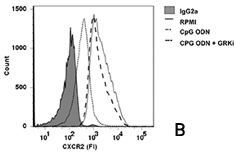
Investigating how TLR9 controls neutrophil recruitment, we also observed that TLR9 activation by type B CpG ODN (CpG-B ODN) induces desensitization of CXCR2 by induction of GRK2. CXCR2 is a G-protein coupled receptor (GPCR) whose presence on leukocytes surface are controlled by specific kinases (termed GRKs). GRK2 phosphorylate serine/threonine residues on GPCRs, leading to receptor internalization and intracellular sorting, which results in either recycling or degradation. Notably, the incubation of neutrophils with a GRK-2 inhibitor (GRKi) prevented the inhibitory effect of CpG-B ODN on CXCL2-induced chemotaxis (Figure 2a) and the down-regulation of CXCR2 (Figure 2b).
In summary, TLR9 is an important receptor in the pathogenesis of sepsis because it contributes to chemokine receptor desensitization in blood neutrophils and impairs the ability of these cells to traffic to sites of infection. We also suggest that development of TLR9-selective antagonists could be useful tools in sepsis management in future.
References:
(1) Silva E, Pedro MA, Sogayar AC, Mohovic T, Silva CL, Janiszewski M et al: Brazilian Sepsis Epidemiological Study (BASES study). Crit Care Med. 2004; 8(4): R251-R260.
(2) Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29(7): 1303-1310.
(3) Reddy RC, Standiford TJ: Effects of sepsis on neutrophil chemotaxis. Curr Opin Hematol. 2010, 17(1): 18-24.
(4) Arraes SM, Freitas MS, Silva SV, Paula Neto HA, Alves-Filho JC, Auxiliadora Martins M: Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood. 2006, 108(9): 2906-2913.
(5) Alves-Filho JC, Freitas A, Souto FO, Spiller F, Paula-Neto H, Silva JS et al: Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A. 2009, 106(10): 4018-4023.
(6) Alves-Filho JC, de Freitas A, Russo M, Cunha FQ: Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006, 34(2): 461-470.
(7) Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr., Auxiliadora-Martins M et al: Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010, 16(6): 708-712.
(8) Rios-Santos F, Alves-Filho JC, Souto FO, Spiller F, Freitas A, Lotufo CM et al: Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am J Respir Crit Care Med. 2007, 175(5): 490-497.
(9) Secher T, Vasseur V, Poisson DM, Mitchell JA, Cunha FQ, Alves-Filho JC, Ryffel B: Crucial role of TNF receptors 1 and 2 in the control of polymicrobial sepsis. J Immunol. 2009, 182(12): 7855-7864.
(10) Freitas A, Alves-Filho JC, Trevelin SC, Spiller F, Suavinha MM, Nascimento DC et al: Divergent role of heme-oxygenase inhibition in the pathogenesis of sepsis. Shock. 2011, 35(6): 550-559.
ULIGINOSIN B, A PHLOROGLUCINOL DERIVATIVE FROM HYPERICUM POLYANTHEMUM, PRODUCE ANTIDEPRESSANT-LIKE EFFECT IN MICE: A NEW MOLECULAR PATTERN PROMISING TO THE DEVELOPMENT OF ANTIDEPRESSANT DRUGS.
Ana C Stein, Alice F Viana, Liz G Müller, Jéssica M Nunes, Eveline D Stolz, Jean C Do Rego, Jean Constentin, Gilsane L von Poser, Stela Maris Kuze Rates. Behavior Brain Research 228 (2012) 66-73. DOI: 10.1016/j.bbr.2011.11.031
New compounds that could improve conventional antidepressant therapies are still needed, since depressive disorders have high incidence in the world population and the treatment of depression with conventional antidepressants provides a complete remission just by 50% of the individuals and presents pronounced side effects, which reduce the patients’compliance to the treatment.
Natural products scaffolds have been well recognized as being privileged structures in terms of their ability to be the basis for successful drugs such as aspirin, opioid analgesics and cardiotonic drugs. Extracts of Hypericum perforatum (St. John’s wort) have long been accepted as a treatment for depression and the components known to play a role in antidepressant activity include phloroglucinol derivatives (hyperforin), naphthodianthrones (hypericin) and the flavonoids (quercitrin). Accepted
In this study, we have demonstrated that cyclohexane extract of Hypericum polyanthemum (POL) and its main phloroglucinol derivative uliginosin B (ULI) (Figure 1) present antidepressant-like activity in rodent forced swimming test (FST). We evaluated the involvement of monoaminergic neurotransmission on the antidepressant-like activity of ULI in vivo and in vitro. POL 90 mg/kg (p.o.) and ULI 10 mg/kg (p.o.) reduced the immobility time in the mice FST (Figure 2) without altering locomotion activity in the open-field test. The combination of sub-effective doses of POL (45 mg/kg, p.o.) and ULI (5 mg/kg p.o.) with sub-effective doses of imipramine (10 mg/kg, p.o.), bupropion (3 mg/kg, p.o.) and fluoxetine (15 mg/kg, p.o.) induced a significant reduction on immobility time in FST (Figure 3 and 4). The pretreatment with SCH23390 (15 µg/kg, s.c., dopamine D1 receptor antagonist) sulpiride (50 mg/kg, i.p., dopamine D2 receptor antagonist), prazosin (1 mg/kg, i.p., α1-adrenoceptor antagonist), yohimbine (1 mg/kg, i.p., α2-adrenoceptor antagonist) and pCPA (100 mg/kg/day, i.p., p-chlorophenilalanine methyl ester, inhibitor of serotonin synthesis, for four consecutive days) before ULI administration (10 mg/kg, p.o.) significantly prevented the anti-immobility effect in FST (Figure 5). ULI was able to inhibit synaptosomal uptake of dopamine (IC50 = 90 ± 38 nM), serotonin (IC50 = 252 ± 13 nM) and noradrenaline (280 ± 48 nM), but it did not bind to any of the monoamine site on the neuronal transporters (Figure 6). These data firstly demonstrated the antidepressant-like effect of POL and ULI, which depends on the activation of the monoaminergic neurotransmission in a different manner from classical antidepressants. In summary, our study showed that dimeric phloglucinol derivatives obtained from species of the genus Hypericum natives to South Brazil could represent a molecular pattern promising to the development of new antidepressant drugs.
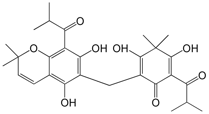
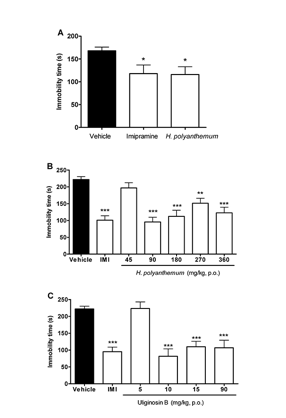
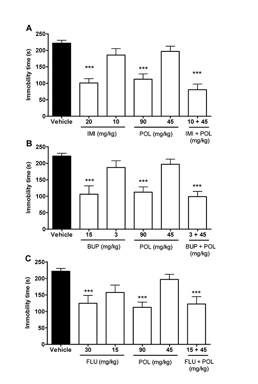
Figure 3 - Effect of the administration of a sub-effective dose of POL (45 mg/kg, p.o.) with sub-effective dose of imipramine (10 mg/kg, p.o.) (A), bupropion (3 mg/kg, p.o.) (B) and fluoxetine (15 mg/kg, p.o.) (C) in the FST. One-Way ANOVA followed by Student Newman-Keuls comparisions ***P<0.001 as compared with the saline-group (vehicle).
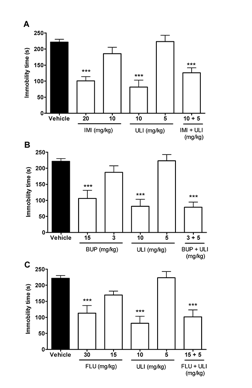
Figure 4 - Effect of the administration of a sub-effective dose of ULI (5 mg/kg, p.o.) with sub-effective dose of imipramine (10 mg/kg, p.o.) (A), bupropion (3 mg/kg, p.o., (B) and fluoxetine (15 mg/kg, p.o.) (C) in the FST. One-Way ANOVA followed by Student Newman-Keuls comparisions ***P<0.001 as compared with the saline-group (vehicle).
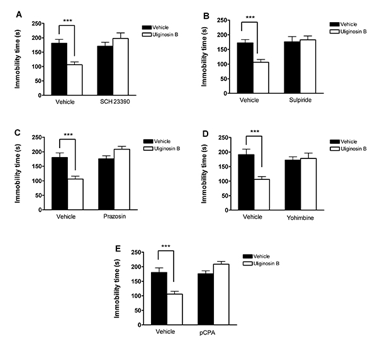
Figure 5 - Effect of pre-treatment of mice with SCH 23390 (15 µg/kg, s.c.)(A), sulpiride (50 mg/kg, i.p. (B), prazosin (1mg/kg, p.o.) (C), yohimbine (1 mg/kg, p.o.) (D) and pCPA (100 mg/kg/day, i.p. (E) on the ULI (10 mg/kg, p.o.) activity in the FST. Two-way ANOVA followed by Student Newman-Keuls comparisions: ***P<0.001 as compared with the control group (vehicle).
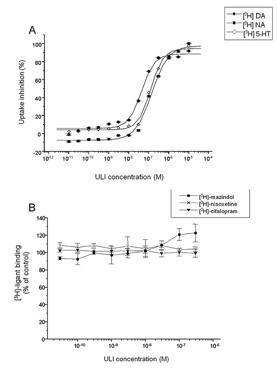
Figure 6. Effect of ULI on [3H]-dopamine (·), [3H]-noradrenalin (n) and [3H]-serotonin (à) synaptosomal uptake (panel A). Effect of ULI on [3H]-mazindol (·), [3H]-nisoxetine (n) and [3H]-citalopram (à) binding to DAT, NAT and SERT, respectively (panel B). The data are presented as percentage of uptake inhibition over basal (mean ± SEM from 4 separated experiments performed in duplicate).
COMMENT FROM THE AUTHORS
This study was carried out in the scope of a big research project aiming at obtention of new neuroactive molecules from Southern Brazilian flora. It illustrates the main steps and challenges imposed when trying to identify active compounds from native plants in the academia context, looking at future drug development. It was supported by a CAPES-COFECUB project (656/09) and developed in cooperation between Brazil and France (Programa de Pós-Graduação em Ciências Farmacêuticas - Universidade Federal do Rio Grande do Sul and Unité de Neuropsychopharmacologie – Université de Rouen). I think this species – Hypericum polyanthemum – was one of the first species native to Southern Brazil that reached intelectual protection property (WO2010092162-A1;INPI: PI0900614-1;PCT/EP2010/051816) and authorization (CGEN/IBAMA 003/2008 - P 02000.001717/2008 – 60) for collecting. It was a really hard - and also exciting –task! But most important we have shown that uliginosin B, a chemotaxonomic marker for species of Hypericum natives to Brazil, represents a useful molecular model for developing new antidepressant drugs.
Stela M. K. Rates.
PLANT DERIVED ALKALOID (-)-CASSINE INDUCES ANTI-INFLAMMATORY AND ANTI-HYPERALGESICS EFFECTS IN BOTH ACUTE AND CHRONIC INFLAMMATORY AND NEUROPATHIC PAIN MODELS.
Kathryn A.B.S. da Silva, Marianne Neves Manjavachi, Ana Flávia Paszcuk, Marcos Pivatto, Claudio Viegas Jr., Vanderlan S. Bolzani, João B. Calixto. Neuropharmacology 62 (2012) 967-977. DOI: doi:10.1016/j.neuropharm.2011.10.002
(-)-Cassine is a major piperidine alkaloid isolated from the flowers, leaves and fruits of Senna spectabilis (syn. of Cassia spectabilis, Fabaceae).1 The ethnopharmacological uses of the plant are associated with its anti-microbial, laxative, anti-ulcerogenic, anti-tumoral activity, analgesic and anti-inflammatory properties.2,3 In early studies we have first reported antinociceptive activity for (-)-spectaline (a co-metabolite of (-) -cassine) in different models of acute pain, including acetic acid, formalin and capsaicin pain models in mice and have also demonstrated that some piperidine alkaloids isolated from Cassia sp., such as (-)-spectaline and (+)-spectaline, show anti-inflammatory, acetylcholinesterase inhibitory and antioxidant activities.4-6 In the present paper we showed the pronounced anti-inflammatory and anti-nociceptive properties of the natural metabolite (-)-cassine, that are probably associated with its ability to inhibit the activation and/or release of various inflammatory mediators such as KC, IL-1β and IL-6. Also, the anti-nociceptive effects of this compound seem to be closely associated with its marked inhibition of PGE2 activity, which occurred mainly through blocking the up regulation of COX-2, MAPK/ERK and NF-kB. Furthermore, we also report the involvement of the TRP family (TRPV1 and TRPA1 receptors) in (-)-cassine anti-nociception effect. Finally, our data also indicate that (-)-cassine has systemic, spinal and supraspinal anti-nociception actions.
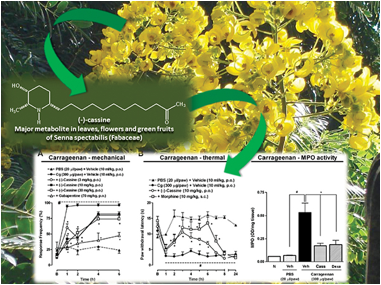
Figure 1. Senna spectabilis, chemical structure of (-)-cassine and its antinociceptive and anti-inflammatory properties.
References:
(1) Pivatto, M. et al. J. Braz. Chem. Soc. 2005, 16, 1431.
(2) Jain, S.C. et al. J. Ethnopharmacol. 1997, 58, 135.
(3) Jafri, M.A. et al. J. Ethnopharmacol. 1999, 66, 355.
(4) Alexandre-Moreira, M.S. et al. Planta Med. 2003, 69, 795.
(5) Viegas Jr., C. et al. J. Nat. Prod. 2004, 67, 908.
(6) Viegas Jr., C. et al. J. Nat. Prod. 2007, 70, 2026.
COMMENTS FROM THE AUTHORS:
This paper is an important contribution to the understanding of the pharmacological profile of (-)-cassine and probably other piperidine alkaloidal analogues metabolites form Senna species. In early studies we have already identified an antinociceptive property of this kind of natural metabolite, but apparently with poor anti-inflammatory activity. In this more complete work we could not only clearly disclose the antinociceptive and anti-inflammatory profile, but also get important evidences about the possible mechanisms of action of this dual pharmacological profile. Based on these informations, we are now working on semi-synthetic derivatives that have showing improved antinociceptive and anti-inflammatory properties.
BIOTRANSFORMATION OF LASSBIO-579 AND PHARMACOLOGICAL EVALUATION OF P-HYDROXYLATED METABOLITE A N-PHENYLPIPERAZINE ANTIPSYCHOTIC LEAD COMPOUND.
Tatiana. F. Gomes; Thais E. T. Pompeu; Daniel A. Rodrigues; François Noël; Ricardo Menegatti; Carolina H. Andrade; José R. Sabino; Eric S. Gil; Teresa Dalla Costa; Andresa H. Betti; Camila B. Antonio; Stela M. K. Rates; Carlos A. M. Fraga; Eliezer J. Barreiro;Valéria de Oliveira. European Journal of Medicinal Chemistry (2012), doi.org/10.1016/j.ejmech.2012.08. 011.V 62, April 2013, 214–221.
Schizophrenia conventionally has been treated with dopamine D2 receptor antagonists such as haloperidol. However, these drugs have little effects on negative and cognitive symptoms and elicit extrapyramidal side effects at therapeutic doses. The introduction of clozapine (1) for treatment-resistant schizophrenia gave rise to a new group of atypical antipsychotics. These drugs exhibit potent antagonism at multiple receptor subtypes, including dopamine and serotonin receptors. However, a significant population of patients is still refractory to treatment, and these new drugs also induce serious side effects [1-3], thus underscoring the importance of developing more effective and safer antipsychotic drugs. In a research program aiming the development of new atypical antipsychotic drugs, with similar clozapine therapeutic profile, high-affinity to D4 receptor with conformational limited flexibility, but devoid of the hematological side effects, a series of heterocyclic N-phenylpiperazine derivatives were planned through molecular hybridization between clozapine (1) and L-741 (2). [1]
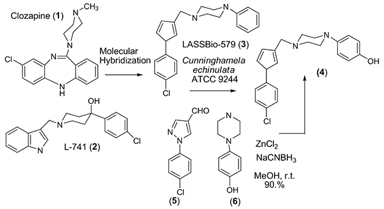
Fig. 1. Compound (3) design, metabolite (4) biosynthesis and organic synthesis.
In vitro and behavioral assays showed that three of these compounds LASSBio-579 (3), act on dopaminergic and serotonergic neurotransmission. As this compound has a very poor oral bioavailability, its activity could depend on until now unknown metabolite(s) that should deserve our attention. A combination of docking and molecular dynamics simulations, we predicted that p-hydroxylation by CYP1A2 would be the main metabolic pathway for the 1-[1-(4-chlorophenyl)-1H-4pyrazolylmethyl] phenylhexahydropiperazine, LASSBio-579 (3). The computational methods show how this antipsychotic lead-compound interacts in active site of CYP1A2. Using our experience with microbial models of mammalian metabolism, [4-5] we produced the p-hydroxylated (4) metabolite of (3) by bioconversion and used it as a reference standard for identifying the metabolite present in plasma after i.p. administration of (3) to rats.

Fig. 2. p-hydroxylated (4) metabolite in silico prediction, in vivo and in vitro production.
The pharmacological activity of the p-hydroxylated (4) metabolite was assayed in binding assays for determining its affinity to relevant receptors for the treatment of schizophrenia. Since our results indicated that (4) is the main metabolite of (3) in vivo, in a rodent model, we decided to determine its affinity for dopamine and 5-HT receptors that have been reported as putative molecular targets for the antipsychotic effect of clozapine (1), our reference drug. For this purpose, we first repeated and extended our previous binding assays with (3) and (1), initially restricted to the D2, 5-HT2A and 5-HT1A receptors [6]. Results indicates that (3), like (1), has a high affinity for the D4 receptor, but not for the 5-HT2C receptor that has been implicated in the mechanism of action of (1). With respect to (4), our data indicate that it has a binding profile very similar to (3), but with a 6-fold higher affinity for the D4 receptor and a 27-fold lower affinity for the 5-HT1A receptor, so that it could be considered as a dual D2-D4 ligand. As side-effects are to be considered when tailoring the drug treatment to the individual schizophrenic patient, we also compared the affinity of (3) and its metabolite (4) for two receptors that have been considered as source of adverse effects of (1), ie the a1B and the muscarinic receptors. Results indicates that both (3) and its main metabolite (4) have a low affinity for the a1B receptor and no affinity for the muscarinic receptor, indicating that these lead compounds could have much less propensity that clozapine to produce adverse effects like postural hypotension and constipation. The compound (4) is a main metabolite of (3) in rat and that it has a high affinity for D2 and D4 receptors indicating that it could participate to the antipsychotic effect of (3) in vivo. Furthermore, the binding studies revealed a more favorable profile than (1) for both (3) and its metabolite (4) regarding two receptors involved in adverse effects.
References
[1]Menegatti, R.; Cunha, A.C.; Ferreira, V.F.; Perreira, E.F.R.; El-Nabawi, A.; Eldefrawi, A.T.; Albuquerque, E.X.; Neves, G.; Rates, S.M.K.; Fraga, C.A.M. and Barreiro, E.J. Bioorg. Med. Chem., 2003, 11, 4807-4813.
[2]Neves, G.; Fenner, R.; Heckler, A.P.; Viana, A. F.; Tasso, L.; Menegatti, R.; Fraga, C.A.M.; Barreiro, E.J.; Dalla Costa, T. and Rates, S.M.K. Braz. J. Med. Biol. Res., 2003, 36, 625-629.
[3] Neves, G.; Kliemann, M.; Betti, A.H.; Conrado, D.J.; Tasso, L.; Fraga, C.A.M.; Barreiro, E.J.; Dalla Costa, T. and Rates, S.M.K. Pharmacol., Biochem. Behav., 2008, 89, 23-30.
[4]Pazini F., Menegatti, R., Sabino J. R., Andrade C. H, Neves G., Rates S. M. K., Noël F., Fraga C. A. M., Barreiro E. J., de Oliveira V. Bioorg Med Chem Lett, 2010, 20, 2888-2891.
[5]Carneiro E.O., Andrade C. H., Braga R.C., Tôrres A. C, Alves R. O., Lião L.M., Fraga C. A. M., Barreiro E. J., de Oliveira V. Bioorg & Med Chem Lett, 2010, 15, 3734-3736.
[6] Neves, G.; Menegatti, R.; Antonio, C.B.; Grazziottin, L.R.; Vieira, R.O.; Rates, S.M.K.; Noël, F.; Barreiro, E.J.; Fraga, C.A.M. Bioorg. Med. Chem. 2010, 18:1925-1935.

VI INCT-INOFAR FOLLOW-UP AND EVALUATION WORKSHOP
With a goal of strengthening the scientific cooperation among its research network and of internally discussing the results achieved in their subprojects that are more advanced in the chain of innovation in drugs and medicines, INCT-INOFAR organizes internal events for follow-up and evaluation.
In May 2012, the Institute welcomed the renowned scientist Dr. Simon Campbell to bring forward the view of the pharmaceutical industry for the evaluation of its research projects. Dr. Campbell is responsible for the discovery of Viagra™ (sildenafil) and of other important medicines for Pfizer laboratories. He came to Brazil exclusively to take part in the VI INCT-INOFAR Follow-Up and Evaluation Workshop.
In the evaluation meeting, which took place on May 14 and 15 2012, in the city of Rio de Janeiro, INCT-INOFAR researchers had the opportunity to present their research projects that are more advanced in the chain of innovation in drugs and medicines, and of being rigorously questioned by the experienced scientist.

INCT-INOFAR researchers met with Dr. Simon Campbell (front row, in a blue shirt and blue tie) to present the main results of their projects of research in new drugs and medicines
In the occasion, Campbell lectured INCT-INOFAR researchers twice. During the opening of the VI Follow-Up and Evaluation Workshop, the scientist talked about the discovery of two medicines where he played important parts – the anti-hypertension drug Norvasc™ (amlodepine) and the famous blue pill, Viagra™ (sildenafil). At the closing conference, Dr. Campbell presented his personal perspective on the future of the pharmaceutical industry.

Simon Campbell presented two lectures at INCT-INOFAR
During the session dedicated to posters, in which 16 INCT-INOFAR research subprojects were presented, Dr. Simon Campbell was amazed at the work done in the bioconversion of human metabolites candidate to new pharmaceutical prototypes developed by the INCT-INOFAR team from the Federal University of Goias (UFG). In reference to this research, the scientist, who was a visiting professor at the University of Sao Paulo (USP) from 1970 to 1972, recognized the importance of INCT-INOFAR for the regional technological development of the country. The project praised by Campbell is coordinated by Professor Valeria de Oliveira, of the Bioconversion Laboratory at UFG.
During a free discussion session, INCT-INOFAR researchers took a turn at questioning Dr. Campbell. Questions related to the choice of therapeutic targets, the scaling of research developed at a college level and the dilemma of patents, like the difficulty of patenting in Brazil and the right time to protect a discovery, were some of the questions asked. At the closing, Campbell praised the multidisciplinary research network established by INCT-INOFAR.

INCT-INOFAR researchers also had the opportunity to question Dr. Simon Campbell on different issues related to the discovery and development of new drugs and medicines.
PROMOTION AND PARTICIPATION IN EVENTS
As an integral part of its institutional routine, INCT-INOFAR organizes, promotes, supports, and takes part in events in its field of research aimed at the innovation in drugs and medicines. A way to actively contribute to the promotion of knowledge in the academic-scientific community, helping Brazil train its human resources and advance in new medicines studies.
Periodically, INCT-INOFAR researchers take part in Congresses, Meetings, Seminars, Symposiums, and Workshops, teaching courses, lecturing, being a part of round tables, as well as other activities. Parallel to these actions, INCT-INOFAR also supports courses and conferences on drugs and medicines. Recognizing the importance of new partnerships, INCT-INOFAR also invests in events that seek out the cooperation of companies, NGOs, and other institutions.
XVIII SUMMER SCHOOL IN MEDICINAL PHARMACEUTICAL CHEMISTRY
http://www.farmacia.ufrj.br/lassbio/xviii_evqfm/
Traditionally organized by the Laboratory of Evaluation and Synthesis of Bioactive Substances (LASSBio™), the Summer School in Medicinal Pharmaceutical Chemistry was incorporated to INCT-INOFAR as an extension activity. The vent, which always takes place at UFRJ during summer academic break, has 5 consecutive days of courses and conferences with renowned Brazilian and foreign experts in the field of Medicinal Pharmaceutical Chemistry. In 2012, from January 23 to 27, the event took place for the 18th time. Since its creation, in 1995, the School has had over 2,500 participants from different parts of Brazil and abroad, and has welcomed renowned scientists responsible for the development of innovative medicines, who personally recounted the stories of their discoveries. To support the presence of Latin American students at the XVIII Summer School, INCT-INOFAR offered scholarship for students from Mercosur countries. In 2012, the event welcomed two young researchers from Uruguay, undergraduate students from the licentiate in Biochemistry at the Universidad de la Republica (Udelar). To celebrate the eighteen years of the Summer School, awarding people with an important scientific trajectory in the field of Medicinal Chemistry, the Organizing Committee for the School created, in 2012, the Camille-Georges Wermuth Medal of Honor in Medicinal Chemistry. Prof. Carlos Alberto Manssour Fraga (http://lattes.cnpq.br/9782159937151139) was awarded the medal. He is an INCT-INOFAR researcher and he was part of one of the first Summer Schools, when he was still applying to be an adjunct professor at UFRJ. Today, Manssour is a Professor at the same institution, working at LASSBio®. Prof. Carlos Manssour received a tribute


Planned to deal with multi and interdisciplinarity of topics, the XVIII Summer School delved into the basics for the courses “Introduction to Medicinal Pharmaceutical Chemistry” and “Metabolism of Drugs and Medication Interactions”, presented the tutorial “Computational Chemistry and Molecular Modeling”, shared foreign knowledge during “Highlights in Medicinal Chemistry”, discussed “Synthesis of Drugs” and went as far as “Intellectual Property and Patents”.
In the programming for the event, internationally renowned lecturers presented important themes in Medicinal Pharmaceutical Chemistry. In 2012, the XVIII Summer School welcomed Prof. Holger Stark from the University Johann Wolfgang Goethe (Germany); Prof. Pier G. Baraldi of the Università di Ferrara (Italy); and Prof. Jos A. S. Cavaleiro of the University of Aveiro (Portugal).

Prof. Baraldi presented Italian Medicinal Chemistry.

Below, Prof. Baraldi (University of Ferrara), Prof. Eliezer (Federal University of Rio de Janeiro) , Prof. Cavaleiro (University of Aveiro), Prof. Stark (Tübingen University) and Prof. Manssour (Federal University of Rio de Janeiro).
35TH ANNUAL MEETING FOR THE BRAZILIAN SOCIETY OF CHEMISTRY
Like in previous editions, INCT-INOFAR was an active part of the 35th Annual Meeting for the Brazilian Society of Chemistry (RASBQ), helping enrich the discussions on Chemistry focused on the development of new drugs and medicines. The 35th RASBQ took place from May 27 to 31, 2012, in the city of Aguas de Lindoia, Sao Paulo. At the event, considered the largest Chemistry event in Latin America, INCT-INOFAR researchers were an important part of the scientific programming. As well as teaching conferences and mini-courses, lecture in theme sessions, and present papers in coordinated sessions and panels, INCT-INOFAR was also part of the 35th RASBQ exposition. At the expo, among corporate stands, with commercial representatives eager to promote their new equipment, the INCT-INOFAR space stood out as it was not selling anything. Its goal was to promote its research work in the area of drugs and medicines, as well as its initiatives for the popularization of science. Those who had the opportunity to visit the stand got to know, and be surprised by, the work done by INCT-INOFAR in the search for new innovative synthesis routes to create generic drugs that are cheaper and use national technology. The visitors could also take home an educational cartoon and a portfolio-magazine that describes the actions in Scientific Awareness & Health Education promoted by INCT-INOFAR. Among the initiatives highlighted in this small portfolio are the educational booklets and puzzles on the correct use of medicines, the video that tells the story of a molecule as it becomes a medicine, as well as an internet portal for the promotion of Pharmaceutical Sciences, the “Portal of Drugs”. The visits to schools, as well as the support to events and the publicizing of INCT-INOFAR research in the media, were also actions that peaked the curiosity of many chemists who were not yet aware of these society actions by the Institute. At the last day of the event, the INCT-INOFAR stand welcomed a group of High School students from Aguas de Lindoia. Thirsty for knowledge, the students, who are at the time of deciding their future careers, asked countless questions that were not only directly related to the Institute, but also to the field of Chemistry. “What is INCT-INOFAR? What research do you develop? How much does a chemist make?” were some of the questions asked by the students who visited the SBQ exposition. Taught the mini-course “Molecular modeling applied to the planning of bioactive compounds” Lectured at the Workshop for the Medicinal Chemistry Division “Interactions between the productive sector and the University in research and innovation in drugs and medicines in Brazil” Launched the book “Organic Chemistry Experiments”, by SBQ Printers Participant in the Special Session with representatives from international scientific societies Lectured at the Symposium “Responsibility, Ethics, and Social Progress” as coordinator for the field of Chemistry at Capes Presented paper in the coordinated session dedicated to “Biological Chemistry and Medicinal Chemistry”. Meeting of INCTs at the 64th SBPC INCT-INOFAR is part of a network that brings together coordinators of the National Institutes of Science and Technology (INCTs) with the goal of discussing governance of the Institutes and strengthening scientific and technological cooperation in research, human resources qualification, and scientific awareness. The network, named I5+, has held its third meeting during the 64th Annual Meeting of the Brazilian Society for the Progress of Science (SBPC), The I5+ meeting took place on July 24 2012, at the Federal University of Maranhao, and was coordinated by Professors Manoel Barral (Vice-President of the CNPq - http://lattes.cnpq.br/0916805360400109 ) and Jailson Bittencourt de Andrade (INCT for Energy and Environment Coordinator -http://lattes.cnpq.br/4049958660392553 ). Representing INCT-INOFAR were its coordinator, Prof. Eliezer J. Barreiro (http://lattes.cnpq.br/5942068988379022 and its scientific superintendent, Prof. Lidia Moreira Lima (http://lattes.cnpq.br/3986190995983234). At the I5+ meeting, subjects like governance, evaluation of the INCTs program, financial resources and scholarships were discussed. Among the main demands from those present were the need to institutionalize the INCTs and the political strengthening of the Program, through explicit initiatives by CNPq. Among the 20 or so INCTs representatives and CNPq technicians were present in the I5+ meeting that took place in São Luis, Maranhão. From September 2 to September 6, 2012, INCT-INOFAR was part of the 22nd International Symposium on Medicinal Chemistry (ISMC 2012), which took place in Berlin, Germany. At the event, promoted by the European Federation of Medicinal Chemistry (EFMC), were present Prof. Eliezer J. Barreiro (http://lattes.cnpq.br/5942068988379022), INCT-INOFAR coordinator, Prof. Lidia Moreira Lima (http://lattes.cnpq.br/3986190995983234), Scientific superintendent, Prof. Angelo da Cunha Pinto (http://lattes.cnpq.br/0061106995455595, CGA member. At the occasion, Institute representatives took advantage of the opportunity to establish new professional contacts and scientific collaborations in Germany. INCT-INOFAR researcher Maria Leticia de Castro Barbosa (http://lattes.cnpq.br/0722721630520953) presented a paper at ISMC 2012, in poster format. At the time, Leticia Barbosa was in an exchange doctoral program at the Interdisciplinary Center for Pharmacogenomics and Pharmaceutical Research (ICEPHA), at the University of Tübingen, under the supervision of Prof. Stefan Laufer. The scientific exchange took place in Germany from October 2011 to September 2012. After this period, the doctoral student returned to Brazil to complete her thesis at the Graduate Program in Chemistry at UFRJ. Silke Bauer (University of Tübingen) and Leticia Barbosa (UFRJ)
With over 8 thousand kilometers of coastal area, the “Blue Rainforest” has in its oceans a pharmacological treasure as rich as the one in the land of the “Green Rainforest”. Still not widely explored by Brazilian scientists, marine life forms are an important source of inspiration for new medications. To discuss the topic and promote new research in the area, the Graduate Program in Pharmacology and Medicinal Chemistry (PPGFQM) from the Biomedical Sciences Institute at UFRJ organized, on October 26, 2012, the International Symposium on the Development of Marine Phytotherapeutics. The event was coordinated by Prof. Roberto Takashi Sudo (http://lattes.cnpq.br/9315809794088995), an associate researcher at INCT-INOFAR. In the programming, conferences and mini-symposiums widened the debate on the chemical-biological diversity found in oceans and also in its surrounding coastal areas. INCT-INOFAR associate professor Patricia Dias Fernandes (http://lattes.cnpq.br/7880284010144634), from the Biomedical Sciences Institute (ICB) at UFRJ, presented her research on the babassu leaf, one of the most important Brazilian palm trees. According to her, the leaf of the plant has been shown to be interesting for its phytotherapeutic use, due to its great antinociceptive effect. That is a noble use for the leaf, as they are not usually used, as only the nut and the oil derived from babassu are marketed. During a whole day of debates, different projects and outlooks on the field of research were presented, among them the PEPMAR project, which is an industry-university partnership with the goal of developing an anticoagulant from the body of the sea cucumber. This research, which is a radical innovation one, and may represent a significant technological leap for the country, is being developed through a public-private partnership between Cristalia Laboratories and the Federal Universities of Rio de Janeiro and Ceará. 

INCT-INOFAR IN THE SCIENTIFIC PROGRAMMING OF THE EVENT
Carlos Mauricio Rabello de Sant’Anna (UFRRJ)
Eliezer J. Barreiro (UFRJ)Barbara Vasconcellos da Silva (UFRJ)
Vanderlan da Silva Bolzani (UNESP- Araraquara)
Luiz Carlos Dias (Unicamp)
Renata Barbosa Lacerda (UFRJ)
I5+ AT SBPC

ISMC 2012


INTERNATIONAL SYMPOSIUM ON THE DEVELOPMENT OF MARINE PHYTOTHERAPEUTICS
BRAZMEDCHEM 2012
SCIENTIFIC AWARENESS AND PROMOTION
Because it believes that the promotion and popularization of Science, Technology, and Innovation are an important factor in building critical thinking skills in the current globalized world, parallel to its laboratory research, INCT-INOFAR coordinates several initiatives in Scientific Awareness & Health Education.
Aware of the potential of children to spread knowledge acquired among friends and family, INCT-INOFAR invests in Health Education initiatives that try to create, among the young, awareness on the rational and safe use of medicines.
Periodically, INCT-INOFAR produces new scientific promotion content about health sciences and creates educational materials focused on the correct use of medicines. By cooperating with the popularization of scientific knowledge inherent to Pharmaceutical Sciences, INCT-INOFAR allows that new vocations be expressed among youth, especially those that are unconnected to their family environments.
With a goal of widening its actions for the promotion and awareness of Pharmaceutical Sciences, INCT-INOFAR created, in April 2012, an Extension Secretary (Av. Carlos Chagas Filho, 373, CCS, bloco K, sala 12, Cidade Universitaria, Rio de Janeiro, RJ). Acting alongside the other Secretaries of the Institute, the Extension Secretary has a goal of spreading INCT-INOFAR health education projects, bringing discussion on the correct use of medicines to public schools.
With a goal of bringing content that is not usually part of school curriculum – the importance of the correct use of medicines – the Drugs and Medicines INCT created, in 2011, the project “INCT-INOFAR in schools”.
In 2012, through the Extension Secretary, the project received the approval of the Municipal Secretary of Education of the Mayor’s Office for the city of Rio de Janeiro, to be carried out in partnership with the 4th Regional Coordination of Education 4thCRE (Ilha do Governador region). The area was chosen due to its proximity with the UFRJ campus, where INCT-INOFAR is headquartered.

With the approval, INCT-INOFAR is now formally authorized to develop its Health Education work in the 177 child education centers and schools of the municipal educational network in the 4th CRE region. To kick off the “INCT-INOFAR in Schools” project, the institute decided to do a pilot project in a public school at the Ramos neighborhood, in Rio de Janeiro.
The institution chosen was the Edmundo Lins Municipal School. With approximately 260 students at the grade school level, the students were divided in 2 shifts to take part of the event promoted by INCT-INOFAR. In the activity done with the children, in June 2011, a couple of INCT-INOFAR pharmacists presented an animated booklet on the correct use of medicines and interacted with the students, encouraging them to ask questions.

- What is the difference between the doctor and the pharmacist?
- Why should we take our medicines at the right time?
- How can we tell if a medicine is a fake?
- Is drinking sugar water a placebo?
VISIT TO THE HOUSE OF SCIENCE
Do you know where the Chemistry is in your house? In the living room, in the bedroom, in the kitchen, or in the bathroom… Those who said in all of those are right! To show that Chemistry is part of our everyday lives, the UFRJ House of Science created, in partnership with the Brazilian Society of Chemistry (SBQ), the “Where is the Chemistry?” show.
At the request of show organizers, INCT-INOFAR loaned its health education materials to delve deeper into questions related to the chemistry behind medications. The content of the INCT-INOFAR booklets inspired an appropriate place to store medicines, in the parents’ bedroom – as it is far from heat, humidity, and away from the reach of children – and the puzzles, inspired in the correct use of medicines, filled the children’s bedroom with play.
To continue its pilot project in health education, INCT-INOFAR invited the students from the Edmundo Lins Municipal School to visit the “Where is the Chemistry?” exposition. From the first grade (formerly literacy class) to the 4th grade, with ages ranging from 5 to 10, separated in small groups, INCT-INOFAR managed to bring all the classes to the exposition.
"WHERE IS THE CHEMISTRY?"

INCT-INOFAR took students from the Edmundo Lins Municipal School to visit the Where is the Chemistry show at the UFRJ House of Science
Inspired by cartoons, the fictitious house from the “Where is the Chemistry?” exposition had all the rooms of a real house, except without walls. Each corner was carefully thought out to promote the science in our daily lives. The guided visit to this fun house, where it was possible to find chemistry in our daily lives, took place on April 26 and 27, and on May 03 and 04, 2012.
In the living room, on the couch, the children received glasses to watch a 3D animation on the chemical history of humankind. “It was like we were inside the TV!” – A boy said. For many children, it was the first time they watched a three dimensional movie.

Living Room
The kitchen is a perfect Chemistry laboratory. Sitting together at the kitchen table, the children all learn about the fantastic world of Chemistry in our daily lives. Why does mommy wrap fruit in newspaper so it ripens faster? And in the fridge, is there Chemistry in there as well?

Kitchen
In the House of Science laundry room, the cleaning products have no brand. They are all generic to show that the name of the manufacturer does not matter. Deep down, they are all the same. They have the same chemical formula to clean.

Laundry Room
But what does Chemistry have to do with our sleep and our dreams? To find out the answer we just need to listen to the stories that come out, curiously, of a large cloud above the children’s bed. To make children more familiar with the complicated names and chemical structure of molecules, the ones that are related to sleep were drawn on the sheets and on the pillows.

Children's Bedroom
In the bathroom, the audience has the opportunity to find out a bit about the history of poop and its uses. A word used as a swear word, a synonym for smelly, in the “Where is the Chemistry?” exposition, children learn why we poop and that it is not a bad thing, it even has a noble use. They just need to open the toilet, put the headphones on, and pay attention to the animation.

Bathroom
In the couple’s bedroom, the audience is invited to lie on the bed to get intimate with mommy and daddy and learn how the chemistry of our body hormones works. An animation on the 7 year itch is curiously shown on the ceiling above their bed.

Bedroom
WHERE DO YOU KEEP MEDICINES?
There are two environments in the house that are not recommended for storing medicines: bathroom and kitchen. The kitchen has fire, and the bathroom has water moisture. Heat and humidity can affect the chemical composition of medicines, which may compromise their therapeutic efficacy. The ideal is to store medications at a place with no temperature changes and that is, of course, far from the reach of children and pets. In the “Where is the Chemistry?” exposition, the medicine box is stored in the couple’s bedroom.

The medicines box is in the couple’s bedroom
“Talking to students in a classroom is one thing, with interactivity it is different. Watching things live, all in a playful way is more conducive to learning. When we returned to the school, we try to contextualize the content in all the different subjects. Everything is useful!”
Heloise Machado Cabral.
Edmundo Lins School teacher

Students return home safely
PORTAL DOS FÁRMACOS
The Portal dos Fármacos (www.portaldosfarmacos.ccs.ufrj.br) is a website maintained by INCT-INOFAR aimed at the promotion and popularization of Pharmaceutical Sciences. Through this portal, INCT-INOFAR publicizes its research activities, in language accessible to laymen, and makes its Health Education materials available. In sync with new trends in scientific journalism, the Portal dos Fármacos has an agenda and journalist coverage of relevant scientific events in the field. Periodically, it publishes new articles and interviews on current topics dealing with innovation in drugs and medicines and in health. It also produces cartoons that critically deal with the irrational use of medicines, proposing conscious alternatives for their use. - Publicizing INCT-INOFAR research activities in language accessible to laymen; - Publishing new articles on current topics surrounding innovation in drugs and medicines and health; - Agenda and Journalistic coverage of the main scientific events in the field; - Download of INCT-INOFAR education booklets dealing with the correct use of medicines. Over one hundred articles, interviews, and reports in the pharmaceutical field have been published in the Portal dos Fármacos since INCT-INOFAR was created in 2009. Among the highlighted topics in the articles published in 2012 are the history of the pharmaceutical profession, recent advances in Medicinal Chemistry, access to essential medications, vaccination campaigns, ethics in scientific research, and development of drugs from marine diversity, among others. Aside from increasing awareness in the population of the correct use of medicines, INCT-INOFAR through the Portal dos Fármacos also support movements aimed at making access to medicines a universal right. On April 2012, the Portal dos Fármacos covered an event organized by Universities Allied for Essential Medications (UAEM) in Brazil. The student activism group fights for social justice and health equity and is present in over 70 Universities throughout the world. At the event, which took place at the University of São Paulo (USP), students from several parts of Brazil discussed how to adequate the movement the reality of our country. The successful campaign by the Yale students inspired the creation of Universities Allied for Essential Medications (UAEM), student activism groups that are now present in over 70 Universities around the world. Fighting for social justice and health equity, UAEM believes that Universities and research institutions have the social responsibility of promoting and managing medical innovation for public interest, ensuring that people, regardless of income, have access to essential medications and other health-related technologies. “During the military dictatorship, the great protest moving force was in the student movement. Today, students need to get back to playing a responsible role in society” – said Eloan Pinheiro, director of Farmanguinhos between 1993 and 2002, who played a fundamental role in the implementation of universal access policies for antiretroviral drugs in Brazil, by standing up to multinational corporations and developing generic AIDS drugs in the state-owned laboratory. To Rachel Kiddell-Monroe, president of the council of UAEM directors, access to medicines is something that may inspire college students to get involved, in a passionate manner, in the cause of health care access. She highlights that many health-related technologies are developed by Universities and research institutes with public funding and then licensed to industry. “The way that intellectual property, licensing, technology transfer, and research priority election policies are created is fundamental for the access to medicines and medical technologies. UAEM depends on the intellectual capacity of students to properly articulate these issues and ensure health care as a universal right” -observed Rachel Kiddell-Monroe. With a multidisciplinary makeup that includes students in the fields of Medicine, Pharmacy, Public Health, Economy, Law, and other related areas, UAEM is a non-profit organization based on the activist movement of University students. Their engagement tries to create in academia, and in future professionals, conscience towards a more proactive attitude to ensure that publically funded discoveries promote global health. Access the full article at: http://www.portaldosfarmacos.ccs.ufrj.br/atualidades_uaem.html In the narrative created by INCT-INOFAR researchers Dr. Lidia Moreira Lima (http://lattes.cnpq.br/3986190995983234), of the Faculty of Pharmacy at UFRJ and Dr. Angelo da Cunha Pinto (http://lattes.cnpq.br/0061106995455595), of the Institute of Chemistry at UFRJ, Joey has a fever and his mother, scared, goes to her sister for advice. She says her son had something similar, and that a friend of hers had recommended a “great” medicine. Joey takes the leftover medicines used by his cousin, and suddenly gets better, but soon is sick again. As they go to a doctor, the family is told of the dangers connected to self-medicating and learns how to correctly use antibiotics. In a playful manner, through the animation of the story of Joey’s illness, INCT-INOFAR explains, in an easily understandable scientific language, how and why bacteria become resistant to antibiotics. It also calls attention to the importance of consulting a doctor, and especially, to rigorously follow the treatment prescribed. With a goal of gathering and publicizing original Organic Chemistry experiments developed throughout the past 10 years, in the Laboratory of Chemistry of Natural Products and Chemical Transformations of the Institute of Chemistry at UFRJ, the researchers associated to INCT-INOFAR Angelo Pinto (http://lattes.cnpq.br/0061106995455595) and Barbara Vasconcellos da Silva (http://lattes.cnpq.br/3874886795138290) organized the book “Experiments in Organic Chemistry”. With relatively simple experiments that use low cost raw materials, the book “Experiments in Organic Chemistry” was published by SBQ as part of the “Chemistry near You” collection. The book was released in May, during the 35th Annual Meeting of the Brazilian Society of Chemistry (SBQ), in Aguas de Lindoia, Sao Paulo. With easily understandable language, the book “Experiments in Organic Chemistry” is aimed at Chemistry teaching and is especially aimed at undergraduate Chemistry students with prior laboratory experience. The book is available online and may be downloaded free at the Brazilian Society of Chemistry (SBQ) website through the link http://www.sbq.org.br/livros.php Funded almost entirely by public resources – from funding agencies like CNPq, Capes, and the State Foundations of Research Support and Sector Funds – Brazilian science must commit to society, to enhance and expand the citizenship of its main financial backer: society. As it tries to cross over from laboratory to society, scientific promotion allows common citizens to have access to knowledge, allowing the creation of a new critical mass capable of evaluation the impact of the social insertion of innovation in their daily lives. Access to quality scientific promotion is fundamental to allow society to demand, politically, the benefits that can be provided by science and technology. Aware of this reality, through its Media Affairs Secretary, INCT-INOFAR invests in actions to promote its most relevant research in the press. As well as accounting to society, the media reports have a goal of creating an interest in pharmaceutical industries and official public laboratories in the development of promising molecules discovered by INCT-INOFAR, that are relevant for Brazilian public health care. It is interesting to notice that, due to the confidentiality and non-disclosure inherent to pharmaceutical area projects, many INCT-INOFAR research projects, especially those in radical innovation, cannot be publicized until its final stages are reached. In the field of generic drugs, two incremental innovation projects by INCT-INOFAR had great repercussion in local and foreign press in the past years. Research makes way for the production of the active principle for these generic drugs at a reduced cost in Brazil. Atorvastatin A continuous use medicine to reduce cholesterol that is widely used, Lipitor™ is the best-selling drug in the world. During the same month where the patent for Lipitor ®/ Pfizer expired in Brazil (December 2010), INCT-INOFAR researchers announced the discovery of a new synthesis route for the production of its active principle, atorvastatin, in a more efficient and economical way. Those in charge of the research were Prof. Luiz Carlos Dias (http://lattes.cnpq.br/2941335797138677) and Dr. Adriano Siqueira Vieira (http://lattes.cnpq.br/8038637214540283) from the Institute of Chemistry of the State University of Campinas (Unicamp). The synthesis route for atorvastatin developed has been patented, and since then, the Institute has tried to negotiate the production of this generic with a Brazilian pharmaceutical company. Sunitinib Recommended to fight certain types of cancers in the kidneys, stomach, and intestines, sunitinib is the active principle for Sutent®/ Pfizer. This is a high cost medicine – around R$ 11,000 a box with 28 pills – that is not yet made available by the Public Health Care System (SUS), and which due to that is the reason for many lawsuits. This research was publicized by INCT-INOFAR in September 2011. The new synthesis route for sunitinib was finished, in September 2011, by Prof. Angelo da Cunha Pinto and by Prof. Barbara Vasconcellos da Silva, from the Institute of Chemistry of the Federal University of Rio de Janeiro (UFRJ). The patent for Sutent® was required by Pfizer laboratories in Brazil in 2005. With this discovery, Brazil can be prepared ahead of time for its production, reducing production costs when the patent for sunitinib expires in the country. As well as publicizing the discovery of new synthesis routes for the production of the active principle of important medicines, INCT-INOFAR also took advantage of this opportunity to bring up in the media the difficulties of producing in Brazil a generic medication with national technology. So far, Brazilian pharmaceutical companies, almost a whole, are limited to formulating and packaging active principles imported from markets such as China, India, and Korea. On May 2012, INCT-INOFAR received a full page at Correio Popular newspaper, for a special report on generic drugs. In an interview with the paper, Prof. Luiz Carlos Dias (Unicamp), researcher responsible for the development of a new synthesis route to produce atorvastatin, spoke about INCT-INOFAR efforts to make Brazil independent from imported raw materials. “National Technology” - Correio Popular Newspaper May 20, 2012 *All the media pieces on INCT-INOFAR research that have been aired or published on the radio, TV, newspapers, magazines, and online media outlets in Brazil and abroad are spontaneous media mentions. Those that can be freely accessed are made available in a specific press clipping area at the Institute’s website. As well as providing press releases for the research in new drugs and medicines carried out by INCT-INOFAR, the Media Affairs Secretary for the Institute also promotes educational materials on the correct use of medicines in the media. “ABC of antibiotics” Revista Ciencia Hoje, n.278. Jan/Fev 2011 An article published on “Science Today” magazine, in 2011, on the cartoon “Joey’s Crew in: the correct use of medicines”, served as reference for 03 questions for a Civil Servant Admittance Exam for technical-administrative workers at the Federal University of Bahia (UFBA).
The exam, which took place on April 22, 2012, at the state of Bahia, was for a laboratory assistant. The position required a grade school education as well as specific knowledge of laboratory practices. On the questions based on the educational booklet produced by INCT-INOFAR, the candidates were required to mark the propositions presented with true (T) or false (F). Since the creation of INCT-INOFAR, the Institute has developed Scientific Awareness & Health Education material. During these four years, two booklets on the correct use of medication have been produced, as well as 10 versions of theme puzzles, a video on the necessary research stages to produce a medication, among other actions. The full portfolio of INCT-INOFAR actions between 2009 and 2011 dealing with scientific awareness and popularization, as well as events and publicizing of its research, is listed in the “INCT-INOFAR 2009 – 2011 Booklet: Scientific Awareness and Health Education”. In a bilingual edition, in Portuguese and English, the booklet is available in print version and online at www.inct-inofar.ccs.ufrj.br/revista. With colorful illustrations and in simple and dynamic language, the booklet “Commandments of the Correct Use of Medicines” warns of the risks associated with the use of medication. In an educational manner, the material provides guidance on the different drug classifications, tells you where and how to store medicines at home, and calls attention to outlandish advertisement by the pharmaceutical industry. Access it at: http://www.portaldosfarmacos.ccs.ufrj.br/download/cartilha_medicamento.pdf In cartoon form, the booklet “Joey’s Crew in: The Correct Use of Antibiotics” calls attention to the risks incurred by the inadequate use of medicines, showing common daily practices that contribute to increasing bacterial resistance, like self-medicating and being “prescribed” drugs by drugstore attendants. The booklet is approved by the Health Surveillance Agency (ANVISA). Access it at: http://www.portaldosfarmacos.ccs.ufrj.br/inct/cartilhas/cartilha_antibiotico.pdf The cartoons published in the Drugs Portal have been transformed into puzzles. Ten different versions of these educational toys have already been produced. The goal is that by assembling the theme puzzles, people are encouraged to reflect on the topics, so that people are more aware of the correct use of medicines. Access the cartoons: http://www.portaldosfarmacos.ccs.ufrj.br/charges.html “LASSBIO 596: from molecule to medication” mixes fiction and science to tell the story of a substance developed by INCT-INOFAR to treat asthma. At 13 minutes long, the video presents the research stages necessary for the medication to reach the drugstore shelves. At each stage, an INCT-INOFAR researcher is shown describing the process. Access it at: http://www.youtube.com/watch?v=wvo6xj6ePPs In the electronic book “Chemistry in Health”, INCT-INOFAR researchers have attempted to explain chemical reactions present in several health situations at a molecular level, dealing with everyday situations and explaining them from a chemical point of view. The issues are shown in a logical sequential order that go from the fecundation of the ova by the spermatozoid, to breastfeeding, to the explanation for the phenomena of puberty through chemical reactions. The e-book is part of the collection “Chemistry in Everyday Life”, produced by the Brazilian Society of Chemistry (SBQ), in celebration of the International Year of Chemistry (AIQ 2011). It is available for free download at http://quimica2011.org.br INCT-INOFAR has produced a hot site to make media clippings on the discovery of a new route for the synthesis of atorvastatin. In it, it is possible to access full versions of media reports, including never before seen interviews with INCT-INOFAR researchers that were publicized in radios, TVs, newspapers, magazines, and online media outlets in Brazil and abroad. Access it at: www.portaldosfarmacos.ccs.ufrj.br/inct/hot_atorvastatina/main.swf Every year, INCT-INOFAR publishes its annual activities report in English, as its main publicity tool in a foreign language. In the report, the main activities of the Institute are listed, highlighting its performance in research, education, scientific awareness, and health education. In extended summary format, the results of the most relevant scientific articles produced by its researchers are presented. On the cover of the reports, a poetic figure gets our attention. Sancho Panza on top of his burro contemplates a constellation of chemical structures, the INCT-INOFAR object of study. In the Miguel de Cervantes allegory, the character is the faithful squire to Don Quixote, and he expresses the search for truth and knowledge in humans. 2009 Annual Activities Report PDF: www.inct-inofar.ccs.ufrj.br/download/aar/2009.pdf Website: www.inct-inofar.ccs.ufrj.br/aar2009/online/main.swf 2010 Annual Activities Report PDF: www.inct-inofar.ccs.ufrj.br/download/aar/2010.pdf Website: www.inct-inofar.ccs.ufrj.br/aar2010/aplicativo/index.html 2011 Annual Activities Report PDF: http://www.inct-inofar.ccs.ufrj.br/download/aar/2011.pdf Website: http://www.inct-inofar.ccs.ufrj.br/aar2011/index.html#inct-inofar 
REAWAKENING A POLITICAL CONSCIENCE IN YOUTH
UAEM believes that Universities can have a positive impact on the lives of populations in developing countries, through humanitarian patenting and licensing strategies, which allow global access to discoveries that were publically funded. The directing of University research priorities, so that they truly meet the interests and needs of most of the population, is also part of the movement’s demands. The incentive to investigations in the field of neglected illnesses is one of its core beliefs.ANIMATION "JOEY'S CREW IN: THE CORRECT USE OF ANTIBIOTICS"
BOOK "EXPERIMENTS IN ORGANIC CHEMISTRY"

INCT-INOFAR IN THE MEDIA*
MEDIA AFFAIRS SECRETARY
Generic Drugs Project

INCT-INOFAR booklet inspires questions at Civil Service Admittance

INCT-INOFAR PORTFOLIO
Portfolio Magazine

Booklet

Cartoon
Puzzle

Video

E-book

Hot site

Annual Activities Report INCT-INOFAR
NATIONAL JOURNALS
1) Estruturas-chave na síntese de antirretrovirais Key molecules in antiretrovirals' synthesis. Mendes, F.M.L., Antunes, A.M.S., Cartaxo, R.J.A. 2012. Revista Virtual de Quimica 4 (3), pp. 329-342.
2) DOI>Constituintes químicos dos galhos de simaba guianensis subesp. ecaudata (cronquist) | [Chemical constituents from stems of simaba guianensis subesp. ecaudata (cronquist)]. De Cássia Saraiva Nunomura, R., Pinto, A.C., Nunomura, S.M., Pohlit, A.M., Amaral, A.C.F. 2012. Química Nova 35 (11), pp. 2153-2158.
3) DOI>Otto R. Gottlieb e as conexões com o Brasil de Ernest Wenkert [Otto R. Gottlieb and Ernest Wenkert's connections with Brazil]. Pinto, A.C., De Da Silva, F.C., Ferreira, V.F. 2012. Química Nova 35 (11), pp. 2317-2323
4) DOI>Química sem fronteiras [Chemistry without borders]. Pinto, A.C., Zucco, C., Galembeck, F., De Andrade, J.B., Vieira, P.C. 2012. Química Nova 35 (10), pp. 2092-2097.
5) DOI>Constituintes químicos do caule de Protium hebetatum (Burseraceae) [Chemical constituents from the stem of Protium hebetatum (Burseraceae)]. Costa, T.O.G., de Almeida, R.A., Koolen, H.H.F., da Silva, F.M.A., Pinto, A.C. 2012. Acta Amazonica 42 (4), pp. 557-560.
6) DOI>Adição de anilinas à naftoquinona em água e em fase sólida [Addition of amines to naphthoquinone in water and solid phase]. Martinez, S.T., Silva, B.V., Pinto, A.C., Ferreira, V.F., De Carvalho Da Silva, F. 2012. Química Nova 35 (4), pp. 858-860.
7) DOI>Educação: Um dever de todos [Education: A duty of all]. Pinto, A.C. 2012. Journal of the Brazilian Chemical Society 23 (7), pp. 1199-1200.
8) A Química Pode Ser Vocação: Basta Melhorá-la no Ensino Médio [Chemistry can be a vocation: Just improve it in high school]. Pinto, A.C. 2012. Revista Virtual de Química 4 (4), pp. 347.
9) A química medicinal de novas moléculas em fase clínica para o tratamento da tuberculose [The medicinal chemistry of novel molecules in clinical trials for tuberculosis treatment]. Branco, F.S.C., Pinto, A.C., Boechat, N. 2012. Revista Virtual de Química 4 (3), pp. 287-328.
10) O REUNI e a expansão dos Cursos de Licenciatura de Química, Física, Matemática e Biologia [The Restructuring and Expansion of Federal Universities (REUNI) and the expansion of degree courses in chemistry, physics, mathematics and biology]. Pinto, A.C. 2012. Revista Virtual de Química 4 (2), pp. 101.
11) A Mata é sua Farmácia - A Pesquisa de Plantas Brasileiras para o Combate de Doenças Tropicais no Século XIX [The forest is his pharmacy - The research of Brazilian plants to combat tropical diseases in the nineteenth century]. Dos Santos, N.P., Pinto, A.C. 2012. Revista Virtual de Química 4 (2), pp. 162-172.
12) Agora é para valer [Now it's for real]. Pinto, A.C. 2012. Revista Virtual de Química 4 (1), pp. 1.
13) DOI>Udp-N-acetilglicosamina enolpiruvil transferase: Determinação dos estados de protonação de resíduos de aminoácidos do sítio ativo pelo método pm6 [Udp-N-acetylglucosamine-enolpyruvyl transferase: Determination of protonation state of active site aminoacid residues by PM6 method]. De Souza, A.X., Sant'Anna, C.M.R. 2012. Química Nova 35 (8), pp. 1522-1526.
14) DOI>Exposição a agrotóxicos e resultados adversos da gravidez: A fragilidade da evidência | [Pesticide exposure and poor pregnancy outcomes: Weaknesses of the evidence]. Paumgartten, F.J.R. 2012. Cadernos de Saúde Pública 28 (10), pp. 2009-2012.
15) DOI>Inviabilidade de uma estratégia de minimização de risco para a sibutramina | [Unfeasibility of a risk mitigation strategy for sibutramine]. Paumgartten, F.J.R. 2012. Revista Brasileira de Psiquiatria 34 (1), pp. 118.
16) DOI>Estudos de biomonitoração do sistema urinário, circulatório e tecidos indicam grande exposição ao bisfenol a | [Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol a]. Vandenberg, L.N., Chahoud, I., Heindel, J.J., Padmanabhan, V., Paumgartten, F.J.R., Schoenfelder, G. 2012. Ciência e Saúde Coletiva 17 (2), pp. 407-434.
17) Detecting fluoride using a plant species as a bioindicator, evaluating the relationship of endocrine and nerve ganglia in small intestine function, and discovering the effects of protein malnourishment and probiotic supplementation in the digestive system of mammalian models.Bonatto, D., Henriques, J.A. 2012. Anais da Academia Brasileira de Ciências 84 (3), pp. 587-588.
18) Discrimination of sugarcane according to cultivar by 1H NMR and chemometric analyses. Alves Filho, E.G., Silva, L.M.A., Choze, R., Lião, L.M., Honda, N.K., Alcantara, G.B. 2012. Journal of the Brazilian Chemical Society 23 (2), pp. 273-279.
19) DOI>Inibição da angiogênese pela própolis verde e efeito angiogênico da L-lisina no câncer de bexiga em ratos | [Angiogenesis inhibition by green propolis and the angiogenic effect of L-lysine on bladder cancer in rats]. Dornelas, C.A., Fechine-Jamacaru, F.V., Albuquerque, I.L., Magalhães, H.I.F., Dias, T.A., Faria, M.H.G., Alves, M.K.S., Rabenhorst, S.H.B., Almeida, P.R.C., Lemos, T.L.G., Castro, J.D.V., Moraes, M.E.A., Moraes, M.O. 2012. Acta Cirurgica Brasileira 27 (8), pp. 529-536.
20) DOI>Quimioprevenção com própolis verde extraído em l-lisina versus promoção da carcinogênese como l- lisina em ratos induzidos ao câncer de bexiga pelo n-butyl-n-[4-hydroxybutyl] nitrosamine (BBN) | [Chemoprevention with green propolis green propolis extracted in l- lysine versus carcinogenesis promotion with l-lysine in n-butyl-n-[4-hydroxybutyl] nitrosamine (bbn) induced rat bladder cancer]. Dornelas, C.A., Fechine-Jamacaru, F.V., Albuquerque, I.L., Magalhães, H.I.F., Silva de Souza, A.J., Alves, L.A., Carvalho de Almeida, P.R., Lemos, T.L.G., Castro, J.D.V., Moraes, M.E.A., Moraes, M.O. 2012. Acta Cirurgica Brasileira 27 (2), pp. 185-192.
21) DOI>In vitro and in vivo antitumor effects of the essential oil from the leaves of guatteria friesiana. Britto, A.C.S., De Oliveira, A.C.A., Henriques, R.M., Cardoso, G.M.B., Bomfim, D.S., Carvalho, A.A., Moraes, M.O., Pessoa, C., Pinheiro, M.L.B., Costa, E.V., Bezerra, D.P. 2012. Planta Médica 78 (5), pp. 409-414.
22) DOI>Modelo experimental de termoterapia ultrassônica em ratos inoculados com tumor de walker-236 | [Experimental model of ultrasound thermotherapy in rats inoculated with walker-236 tumor]. Morano, J.A.C.O.D., Cordeiro, N., Guimarães, S.B., Fechine-Jamacaru, F.V., de Vasconcelos, P.R.L., de Moraes Filho, M.O. 2012. Acta Cirurgica Brasileira 27 (1), pp. 201-2017.
23) DOI>Segurança do tratamento com ciclosporina em um modelo pré-clínico de tumor cerebral experimental em ratos | [Cyclosporin safety in a simplified rat brain tumor implantation model]. Felix, F.H.C., Fontenele, J.B., Teles, M.G., Neto, J.E.B., Santiago, M.H.A.M., Filho, R.L.P., de Menezes, D.B., Viana,
24) DOI>Sensitive method for determination of piplartine, an alkaloid amide from Piper species, in rat plasma samples by liquid chromatography-tandem mass spectrometry. Bezerra, D.P., Pessoa, C., Moraes, M.O., Costa-Lotufo, L.V., Gouvea, D.R., Jabor, V.A.P., Lopes, N.P., Borges, K.B., Lima, M.A.S., Silveira, E.R. 2012. Química Nova 35 (3), pp. 460-465.
25) LinkAvaliação in vitro do potencial biológico da Salvia officinalis L. em células tumorais | [In vitro evaluation of the biological potential of Salvia officinalis L. in tumor cells]. Garcia, C.S.C., Lambert, A.P.F., Henriques, J.A.P., Ely, M.R. 2012. Scientia Medica 22 (3), pp. 131-137.
26) DOI>Principais conclusões do workshop conjunto dos Programas FAPESP BIOTA-BIOEN-mudanças Climáticas: Ciência e políticas públicas para uma economia mais verde, no contexto da RIO+20 | [Main conclusions of the joint FAPESP BIOTA-BIOEN-climate change workshop: Science and policy for a greener economy in the context of RIO+20]. Joly, C.A., Berlinck, R.G.S., Bolzani, V.S., Haddad, C.F.B., de Oliveira, M.C., van Sluys, M.-A., Souza, G.M., Verdade, L.M., Victoria, R.L., 2012. Biota Neotropica 12 (2), pp. 19-21.
INTERNATIONAL JOURNALS
1) DOI>Multicriteria mapping of stakeholder preferences for the sustainability of the Brazilian program for biodiesel production and use. Mendes, P.A., Barros, A.K., d'Avila, L.A., Antunes, A.M. 2012. Environmental Progress and Sustainable Energy 27 (4).Article in Press.
2) DOI>Use of patent applications as a tool for technology development investigations on ethanol production from lignocellulosic biomass in Brazil. Schlittler, L.A.F.S., Antunes, A.M.S., Pereira Jr., N. 2012. Journal of Technology Management and Innovation 7 (3), pp. 80-90.
3)http://www.chemistry-today.com/testata.asp?id_testata=267&folder=backissueThe pharmaceutical industry in Brazil: Is innovation the next step for the domestic industry?Filho, P.L.P., Antunes, A., Bomtempo, J.V. 2012. Chimica Oggi/Chemistry Today 30 (5), pp. 87-89.
4) DOI>Knowledge management and analysis of scientific biotechnology trends in Venezuela. de Santana, M.F.E., Martínez, R.G., Pereira Jr., N., Antunes, A.M.S. 2012. Journal of Technology Management and Innovation 7 (1), pp. 144-158.
5) DOI>A aventura e a beleza da química [The adventure and beauty of chemistry] Pinto, A.C. 2012. Journal of the Brazilian Chemical Society 23 (9), pp. 1577-1578.
6) DOI>Sem educação básica de qualidade não há futuro [There is no future without good primary education]. Pinto, A.C. 2012. Journal of the Brazilian Chemical Society 23 (8), pp. 1409-1410.
7) DOI>O ensino médio de química: O que fazer para melhorá-lo? [High school chemistry teaching: How to improve it?]. Pinto, A.C. 2012. Journal of the Brazilian Chemical Society 23 (6), pp. 985-986.
8) DOI>Preparation, characterisation and evaluation of brazilian clay-based catalysts for use in esterification reactions. Rezende, M.J.C., Pereira, M.S.C., Santos, G.F.N., Aroeira, G.O.P., Albuquerque Jr., T.C., Suarez, P.A.Z., Pinto, A.C. 2012. Journal of the Brazilian Chemical Society 23 (7), pp. 1209-1215.
9) DOI>Mesophase evolution in heat-treated solid petroleum pitches. Lima, A.L.D.S., Lima, K.D.S.C., França, T.C.C., Tavares, M.I.B., Da Silva San-Gil, R.A., Eberlin, M.N., Pinto, A.C. 2012. Journal of the Brazilian Chemical Society 23 (7), pp. 1355-1371.
10) DOI>A Sociedade Brasileira de Química e o Ano Internacional da Química [The Brazilian chemical society and the International year of chemistry]. Pinto, A.C., Galembeck, F., De Andrade, J.B. 2012. Journal of the Brazilian Chemical Society 23 (3), pp. 373-376.
11) DOI>Comparative in vitro and in vivo antimalarial activity of the indole alkaloids ellipticine, olivacine, cryptolepine and a synthetic cryptolepine analog. Rocha E Silva, L.F., Montoia, A., Amorim, R.C.N., Melo, M.R., Henrique, M.C., Nunomura, S.M., Costa, M.R.F., Andrade Neto, V.F., Costa, D.S., Dantas, G., Lavrado, J., Moreira, R., Paulo, A., Pinto, A.C., Tadei, W.P., Zacardi, R.S., Eberlin, M.N., Pohlit, A.M. 2012. Phytomedicine 20 (1), pp. 71-76.
12) DOI>Synthesis of carbohydrate-based naphthoquinones and their substituted phenylhydrazono derivatives as anticancer agents. Campos, V.R., Dos Santos, E.A., Ferreira, V.F., Montenegro, R.C., De Souza, M.C.B.V., Costa-Lotufo, L.V., De Moraes, M.O., Regufe, A.K.P., Jordão , A.K., Pinto, A.C., Resende, J.A.L.C., Cunha, A.C. 2012. RSC Advances 2 (30), pp. 11438-11448.
13) DOI>Selective cytotoxicity and apoptosis induction in glioma cell lines by 5-oxygenated-6,7-methylenedioxycoumarins from Pterocaulon species. Vianna, D.R., Hamerski, L., Figueiró, F., Bernardi, A., Visentin, L.C., Pires, E.N.S., Teixeira, H.F., Salbego, C.G., Eifler-Lima, V.L., Battastini, A.M.O., von Poser, G.L., Pinto, A.C. 2012. European Journal of Medicinal Chemistry 57, pp. 268-274.
14) DOI>Synthesis of porphyrin indolin-2-one conjugates via palladium-catalyzed amination reactions. Menezes, J.C.J.M.D.S., Pereira, A.M.V.M., Neves, M.G.P.M.S., Silva, A.M.S., Santos, S.M., Martinez, S.T., Silva, B.V., Pinto, A.C., Cavaleiro, J.A.S. 2012.Tetrahedron 68 (39), pp. 8330-8339.
15) DOI>Proximity to competitors changes secondary metabolites of non-indigenous cup corals, Tubastraea spp., in the southwest Atlantic. Lages, B.G., Fleury, B.G., Hovell, A.M.C., Rezende, C.M., Pinto, A.C., Creed, J.C.2012. Marine Biology 159 (7), pp. 1551-1559.
16) DOI>New trifluoromethyl triazolopyrimidines as Anti-Plasmodium falciparum agents. Boechat, N., Pinheiro, L.C.S., Silva, T.S., Aguiar, A.C.C., Carvalho, A.S., Bastos, M.M., Costa, C.C.P., Pinheiro, S., Pinto, A.C., Mendonça, J.S., Dutra, K.D.B., Valverde, A.L., Santos-Filho, O.A., Ceravolo, I.P., Krettli, A.U. 2012. Molecules 17 (7), pp. 8285-8302.
17) DOI>Structures of 1-(substituted-phenyl)-4-hydroxymethyland-4-fluoromethyl-1,2, 3-triazoles. Boechat, N., De Lourdes G. Ferreira, M., Bastos, M.M., Pinto, A.C., Da Silva, G.P., Costa, C.C.P., Wardell, S.M.S.V., Wardell, J.L. 2012. Zeitschrift fur Kristallographie 227 (6), pp. 369-378.
18) DOI>Terpenes from copaifera demonstrated in vitro antiparasitic and synergic activity. Izumi, E., Ueda-Nakamura, T., Veiga, V.F., Pinto, A.C., Nakamura, C.V. 2012. Journal of Medicinal Chemistry 55 (7), pp. 2994-3001.
19) DOI>Discovery of Novel Orally Active Anti-Inflammatory N-Phenylpyrazolyl-N-Glycinyl-Hydrazone Derivatives That Inhibit TNF-α Production. Lacerda, R.B., da Silva, L.L., de Lima, C.K.F., Miguez, E., Miranda, A.L.P., Laufer, S.A., Barreiro, E.J., Fraga, C.A.M. 2012. PLoS ONE 7 (10) , art. no. e46925.
20) DOI>Phenylpiperazine derivatives: A patent review (2006 - Present). Maia, R.D.C., Tesch, R., Fraga, C.A.M. 2012. Expert Opinion on Therapeutic Patents 22 (10), pp. 1169-1178.
21) DOI>Design, synthesis, and pharmacological evaluation of N-acylhydrazones and novel conformationally constrained compounds as selective and potent orally active phosphodiesterase-4 inhibitors. Kümmerle, A.E., Schmitt, M., Cardozo, S.V.S., Lugnier, C., Villa, P., Lopes, A.B., Romeiro, N.C., Justiniano, H., Martins, M.A., Fraga, C.A.M., Bourguignon, J-J., Barreiro, E.J. 2012. Journal of Medicinal Chemistry 55 (17), pp. 7525-7545.
22) DOI>Vasodilator and antihypertensive effects of a novel N-acylhydrazone derivative mediated by the inhibition of L-type Ca 2+ channels. Pereira, S.L., Kummerle, A.E., Fraga, C.A.M., Barreiro, E.J., Sudo, R.T., Zapata-Sudo, G. 2012. Fundamental and Clinical Pharmacology.Article in Press.
23) DOI>Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A 2A adenosine receptor. Leal, C.M., Pereira, S.L., Kümmerle, A.E., Leal, D.M., Tesch, R., De Sant'Anna, C.M.R., Fraga, C.A.M., Barreiro, E.J., Sudo, R.T., Zapata-Sudo, G.2012.European Journal of Medicinal Chemistry 55, pp. 49-57.
24) DOI>Design and synthesis of new (E)-cinnamic N-acylhydrazones as potent antitrypanosomal agents. Carvalho, S.A., Feitosa, L.O., Soares, M., Costa, T.E.M.M., Henriques, M.G., Salomão, K., De Castro, S.L., Kaiser, M., Brun, R., Wardell, J.L., Wardell, S.M.S.V., Trossini, G.H.G., Andricopulo, A.D., Silva, E.F., Fraga, C.A.M. 2012. European Journal of Medicinal Chemistry 54, pp. 512-521.
25) DOI>(2E)-N′-[(E)-Benzylidene]-3-phenylprop-2-enohydrazide from synchrotron radiation. Carvalho, S.A., Silva, E.F.D., Fraga, C.A.M., Wardell, S.M.S.V., Wardell, J.L., Tiekink, E.R.T. 2012. Acta Crystallographica Section E: Structure Reports Online 68 (7), pp. o2255-o2256.
26)DOI>(2E)-N′-[(E)-2-Hydroxybenzylidene]-3-phenylprop-2-enohydrazide. Carvalho, S.A., Silva, E.F.D., Fraga, C.A.M., Wardell, S.M.S.V., Wardell, J.L., Tiekink, E.R.T. 2012. Acta Crystallographica Section E: Structure Reports Online 68 (7), pp. o2253-o2254.
27) DOI>Synthesis and characterization of the atropisomeric relationships of a substituted N-phenyl-bipyrazole derivative with anti-inflammatory properties. Veloso, M.P., Romeiro, N.C., Silva, G.M.S., Alves, H.D.M., Doriguetto, A.C., Ellena, J., Miranda, A.L.P., Barreiro, E.J., Fraga, C.A.M. 2012. Chirality 24 (6), pp. 463-470.
28) DOI>Combination of docking, molecular dynamics and quantum mechanical calculations for metabolism prediction of 3,4-methylenedioxybenzoyl-2- thienylhydrazone. Braga, R.C., Alves, V.M., Fraga, C.A.M., Barreiro, E.J., De Oliveira, V., Andrade, C.H. 2012. Journal of Molecular Modeling 18 (5), pp. 2065-2078.
29) DOI>Novel furfurylidene N-acylhydrazones derived from natural safrole: Discovery of LASSBio-1215, a new potent antiplatelet prototype. Rodrigues, A.P.C., Costa, L.M.M., Santos, B.L.R., Maia, R.C., Miranda, A.L.P., Barreiro, E.J., Fraga, C.A.M. 2012. Journal of Enzyme Inhibition and Medicinal Chemistry 27 (1), pp. 101-109.
30) DOI>Dialkylphosphorylhydrazones as potent tyrosinase inhibitors. Caixeiro, J.M.R., Gonçalves, V.T., De Oliveira, M.C.C., Sant'Anna, C.M.R., Rumjanek, V.M., DaCosta, J.B.N. 2012. Journal of the Brazilian Chemical Society 23 (5), pp. 804-809.
31) DOI>Solvent-free synthesis, DNA-topoisomerase II activity and molecular docking study of new asymmetrically N,N′-substituted ureas. Andressa, E.-S., Rodrigues-Santos, C.E., De Nigris Del Cistia, C., Da Silva, D.R., Sant'Anna, C.M.R., Echevarria, A. 2012. Molecules 17 (11), pp. 12882-12894.
32) DOI>Structural insights into cholinesterases inhibition by harmane β-carbolinium derivatives: A kinetics - Molecular modeling approach. Torres, J.M., Lira, A.F., Silva, D.R., Guzzo, L.M., Sant'Anna, C.M.R., Kümmerle, A.E., Rumjanek, V.M.2012. Phytochemistry 81, pp. 24-30.
33) DOI>Investigation of trypanothione reductase inhibitory activity by 1,3,4-thiadiazolium-2-aminide derivatives and molecular docking studies. Rodrigues, R.F., Castro-Pinto, D., Echevarria, A., Dos Reis, C.M., Del Cistia, C.N., Sant'Anna, C.M.R., Teixeira, F., Castro, H., Canto-Cavalheiro, M., Leon, L.L., Tomás, A. 2012.Bioorganic and Medicinal Chemistry 20 (5), pp. 1760-1766.
34) DOI>Molecular docking and molecular dynamic studies of semi-synthetic piperidine alkaloids as acetylcholinesterase inhibitors. Danuello, A., Romeiro, N.C., Giesel, G.M., Pivatto, M., Viegas Jr., C., Verli, H., Barreiro, E.J., Fraga, C.A.M., Castro, N.G., Bolzani, V.S. 2012. Journal of the Brazilian Chemical Society 23 (1), pp. 163-170.
35) DOI>Natural products from Brazilian biodiversity as a source of new models for medicinal chemistry. Bolzani, V.S., Valli, M., Pivatto, M., Viegas Jr., C. 2012. Pure and Applied Chemistry 84 (9), pp. 1837-1846.
36) DOI>(±)-2,2-Dimethyl-5-oxotetra-hydro-furan-3-carb-oxy-lic acid (terebic acid): A racemic layered structure. Santos, L.M., Legendre, A.O., Villis, P.C.M., Viegas Jr., C., Doriguetto, A.C. 2012. Acta Crystallographica Section C: Crystal Structure Communications 68 (8), pp. o294-o297.
37) DOI>4-{[4-(Hydroxymethyl)piperidin-1-yl]methyl}phenol. Simões, M.C.R., Landre, I.M.R., Moreira, M.S., Viegas Jr., C., Doriguetto, A.C. 2012. Acta Crystallographica Section E: Structure Reports Online 68 (7), pp. o2275-o2276.
38) DOI>The genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and pharmacological characteristics. Baldim Zanin, J.L., De Carvalho, B.A., Martineli, P.S., Dos Santos, M.H., Lago, J.H.G., Sartorelli, P., Viegas Jr., C., Soares, M.G. 2012. Molecules 17 (7), pp. 7887-7902.
39) DOI>Isolation and evaluation of the antioxidant activity of phenolic constituents of the Garcinia brasiliensis epicarp. Gontijo, V.S., De Souza, T.C., Rosa, I.A., Soares, M.G., Da Silva, M.A., Vilegas, W., Viegas Jr., C., Dos Santos, M.H. 2012. Food Chemistry 132 (3), pp. 1230-1235.
40) DOI>Semisynthesis and antimicrobial activity of novel guttiferone-A derivatives. Dias, K.S.T., Januário, J.P., D'Dego, J.L., Dias, A.L.T., Dos Santos, M.H., Camps, I., Coelho, L.F.L., Viegas Jr., C.2012. Bioorganic and Medicinal Chemistry 20 (8), pp. 2713-2720.
41) DOI>Plant derived alkaloid (-)-cassine induces anti-inflammatory and anti-hyperalgesics effects in both acute and chronic inflammatory and neuropathic pain models. Da Silva, K.A.B.S., Manjavachi, M.N., Paszcuk, A.F., Pivatto, M., Viegas Jr., C., Bolzani, V.S., Calixto, J.B.2012. Neuropharmacology 62 (2), pp. 967-977.
42) DOI>Effect of endogenous hydrogen sulfide inhibition on structural and functional renal disturbances induced by gentamicins. Francescato, H.D.C., Chierice, J.R.A., Marin, E.C.S., Cunha, F.Q., Costa, R.S., Silva, C.G.A., Coimbra, T.M. 2012. Brazilian Journal of Medical and Biological Research 45 (3), pp. 244-249.
43) DOI>Role of KATP channels and TRPV1 receptors in hydrogen sulfide-enhanced gastric emptying of liquid in awake mice. Medeiros, J.V.R., Bezerra, V.H., Lucetti, L.T., Lima-Júnior, R.C.P., Barbosa, A.L.R., Tavares, B.M., Magalhães, P.J.C., Santos, A.A., Cunha, F.Q., Soares, P.M.G., Souza, M.H.L.P. 2012. European Journal of Pharmacology 693 (1-3), pp. 57-63.
44) DOI>Recombinant migration inhibitory factor induces nitric oxide synthase in murine macrophages (The Journal of Immunology (1993) 150, (1908-1912)). Cunha, F.Q., Weiser, W.Y., David, J.R., Moss, D.W., Moncada, S., Liew, F.Y.2012. Journal of Immunology 189 (6), pp. 3260.
45) DOI>Toll-like receptor 9 activation in neutrophils impairs chemotaxis and reduces sepsis outcome. Trevelin, S.C., Alves-Filho, J.C., Sônego, F., Turato, W., Nascimento, D.C., Souto, F.O., Cunha, T.M., Gazzinelli, R.T., Cunha, F.Q. 2012. Critical Care Medicine 40 (9), pp. 2631-2637.
46) DOI>Docking, synthesis and pharmacological activity of novel urea-derivatives designed as p38 MAPK inhibitors. De Oliveira Lopes, R., Romeiro, N.C., De Lima, C.K.F., Louback Da Silva, L., Palhares De Miranda, A.L., Nascimento, P.G.B.D., Cunha, F.Q., Barreiro, E.J., Lima, L.M. 2012. European Journal of Medicinal Chemistry 54, pp. 264-271.
47) DOI>α1-acid glycoprotein decreases neutrophil migration and increases susceptibility to sepsis in diabetic mice. Spiller, F., Carlos, D., Souto, F.O., De Freitas, A., Soares, F.S., Vieira, S.M., Paula, F.J.A., Alves-Filho, J.C., Cunha, F.Q. 2012. Diabetes 61 (6), pp. 1584-1591.
48) DOI>Activation pattern of neutrophils from blood of elderly individuals with Candida-related denture stomatitis. Gasparoto, T.H., De Oliveira, C.E., Vieira, N.A., Porto, V.C., Cunha, F.Q., Garlet, G.P., Campanelli, A.P., Lara, V.S. 2012. European Journal of Clinical Microbiology and Infectious Diseases 31 (6), pp. 1271-1277.
49)DOI>Neutrophil paralysis in plasmodium vivax malaria. de Leoratti, F.M.S., Trevelin, S.C., Cunha, F.Q., Rocha, B.C., Costa, P.A.C., Gravina, H.D., Tada, M.S., Pereira, D.B., Golenbock, D.T., Antonelli, L.R.V., Gazzinelli, R.T. 2012. PLoS Neglected Tropical Diseases 6 (6), art. no. e1710.
50) DOI>Kaurenoic acid from Sphagneticola trilobata inhibits inflammatory pain: Effect on cytokine production and activation of the NO-cyclic GMP-protein kinase G-ATP-sensitive potassium channel signaling pathway. Mizokami, S.S., Arakawa, N.S., Ambrosio, S.R., Zarpelon, A.C., Casagrande, R., Cunha, T.M., Ferreira, S.H., Cunha, F.Q., Verri, W.A. 2012. Journal of Natural Products 75 (5), pp. 896-904.
51) DOI>Flavonoids as anti-inflammatory and analgesic drugs: Mechanisms of action and perspectives in the development of pharmaceutical forms. Verri Jr., W.A., Vicentini, F.T.M.C., Baracat, M.M., Georgetti, S.R., Cardoso, R.D.R., Cunha, T.M., Ferreira, S.H., Cunha, F.Q., Fonseca, M.J.V., Casagrande, R. 2012. Studies in Natural Products Chemistry 36, pp. 297-330.
52) DOI>Joint NOD2/RIPK2 signaling regulates IL-17 axis and contributes to the development of experimental arthritis. Vieira, S.M., Cunha, T.M., França, R.F.O., Pinto, L.G., Talbot, J., Turato, W.M., Lemos, H.P., Lima, J.B., Verri, W.A., Almeida, S.C.L., Ferreira, S.H., Louzada-Junior, P., Zamboni, D.S., Cunha, F.Q. 2012. Journal of Immunology 188 (10), pp. 5116-5122.
53) DOI>Trypanosoma cruzi adjuvants potentiate T cell-mediated immunity induced by a NY-ESO-1 based antitumor vaccine. Junqueira, C., Guerrero, A.T., Galvão-Filho, B., Andrade, W.A., Salgado, A.P.C., Cunha, T.M., Ropert, C., Campos, M.A., Penido, M.L.O., Mendonça-Previato, L., Previato, J.O., Ritter, G., Cunha, F.Q., Gazzinelli, R.T. 2012. PLoS ONE 7 (5), art.no. e36245.
54) DOI>Acetic acid- and phenyl-p-benzoquinone-induced overt pain-like behavior depends on spinal activation of MAP kinases, PI 3K and microglia in mice. Pavao-De-Souza, G.F., Zarpelon, A.C., Tedeschi, G.C., Mizokami, S.S., Sanson, J.S., Cunha, T.M., Ferreira, S.H., Cunha, F.Q., Casagrande, R., Verri Jr., W.A. 2012. Pharmacology Biochemistry and Behavior 101 (3), pp. 320-328.
55) DOI>Bosentan, an endothelin receptor antagonist, ameliorates collagen-induced arthritis: The role of TNF-α in the induction of endothelin system genes. Donate, P.B., Cunha, T.M., Verri Jr., W.A., Junta, C.M., Lima, F.O., Vieira, S.M., Peres, R.S., Bombonato-Prado, K.F., Louzada, P., Ferreira, S.H., Donadi, E.A., Passos, G.A.S., Cunha, F.Q. 2012. Inflammation Research 61 (4), pp. 337-348.
56) DOI>The protein LJM 111 from Lutzomyia longipalpis Salivary Gland Extract (SGE) accounts for the SGE-inhibitory effects upon inflammatory parameters in experimental arthritis model. Grespan, R., Lemos, H.P., Carregaro, V., Verri Jr., W.A., Souto, F.O., De Oliveira, C.J.F., Teixeira, C., Ribeiro, J.M., Valenzuela, J.G., Cunha, F.Q. 2012. International Immunopharmacology 12 (4), pp. 603-610.
57) DOI>Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: Role of cytokines on inducible nitric oxide synthase activation. Lima Jr., R.C.P., Figueiredo, A.A., Freitas, H.C., Melo, M.L.P., Wong, D.V.T., Leite, C.A.V.G., Medeiros, R.P., Marques-Neto, R.D., Vale, M.L., Brito, G.A.C., Oriá, R.B., Souza, M.H.L.P., Cunha, F.Q., Ribeiro, R.A. 2012. Cancer Chemotherapy and Pharmacology 69 (4), pp. 931-942.
58) DOI>Stimulation of peripheral Kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kγ/AKT/nNOS/NO signaling pathway. Cunha, T.M., Souza, G.R., Domingues, A.C., Carreira, E.U., Lotufo, C.M., Funez, M.I., Verri, W.A., Cunha, F.Q., Ferreira, S.H. 2012. Molecular Pain 8, art.no. 10.
59) DOI>Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFα and CXCL1/CXCR2 in mice. Zarpelon, A.C., Pinto, L.G., Cunha, T.M., Vieira, S.M., Carregaro, V., Souza, G.R., Silva, J.S., Ferreira, S.H., Cunha, F.Q., Verri, W.A. 2012. Canadian Journal of Physiology and Pharmacology 90 (2), pp. 187-199.
60) DOI>NLRP3 inflammasome-mediated neutrophil recruitment and hy
pernociception depend on leukotriene B4 in a murine model of gout. Amaral, F.A., Costa, V.V., Tavares, L.D., Sachs, D., Coelho, F.M., Fagundes, C.T., Soriani, F.M., Silveira, T.N., Cunha, L.D., Zamboni, D.S., Quesniaux, V., Peres, R.S., Cunha, T.M., Cunha, F.Q., Ryffel, B., Souza, D.G., Teixeira, M.M. 2012. Arthritis and Rheumatism 64 (2), pp. 474-484.
61) DOI>NADPH phagocyte oxidase knockout mice control trypanosoma cruzi proliferation, but develop circulatory collapse and succumb to infection. Santiago, H.C., Lombana, C.Z.G., Macedo, J.P., Utsch, L., Tafuri, W.L., Campagnole-Santos, M.J., Alves, R.O., Alves-Filho, J.C., Romanha, A.J., Cunha, F.Q., Teixeira, M.M., Radi, R., Vieira, L.Q. 2012. PLoS Neglected Tropical Diseases 6 (2), art. no. e1492.
62) DOI>A crucial role for IL-6 in the CNS of rats during fever induced by the injection of live E. coli. Soares, D.M., Figueiredo, M.J., Martins, J.M., Machado, R.R., Sorgi, C., Faciolli, L.H., Alves-Filho, J.C., Cunha, F.Q., Souza, G.E.P. 2012. Medical Microbiology and Immunology 201 (1), pp. 47-60.
63) DOI>Interleukin-4 modulates the inflammatory response in ifosfamide-induced hemorrhagic cystitis. Macedo, F.Y.B., Mourão, L.T.C., Freitas, H.C., Lima Jr., R.C.P., Wong, D.V.T., Oriá, R.B., Vale, M.L., Brito, G.A.C., Cunha, F.Q., Ribeiro, R.A. 2012. Inflammation 35 (1), pp. 297-307.
64) DOI>Role of CCR2 in orthodontic tooth movement. Taddei, S.R.D.A., Andrade Jr., I., Queiroz-Junior, C.M., Garlet, T.P., Garlet, G.P., Cunha, F.D.Q., Teixeira, M.M., Da Silva, T.A. 2012. American Journal of Orthodontics and Dentofacial Orthopedics 141 (2), pp. 153-160.
65) DOI>Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Czepielewski, R.S., Porto, B.N., Rizzo, L.B., Roesler, R., Abujamra, A.L., Pinto, L.G., Schwartsmann, G., Cunha, F.Q., Bonorino, C. 2012. Proceedings of the National Academy of Sciences of the United States of America 109 (2), pp. 547-552.
66) DOI>PPAR-γ agonists, mainly 15d-PGJ 2, reduce eosinophil recruitment following allergen challenge. Farnesi-de-Assunção, T.S., Alves, C.F., Carregaro, V., de Oliveira, J.R., da Silva, C.A.T., Cheraim, A.B., Cunha, F.Q., Napimoga, M.H. 2012. Cellular Immunology 273 (1), pp. 23-29.
67) DOI>Inhibition of iNOS induces antidepressant-like effects in mice: Pharmacological and genetic evidence. Montezuma, K., Biojone, C., Lisboa, S.F., Cunha, F.Q., Guimarães, F.S., Joca, S.R.L. 2012. Neuropharmacology 62 (1), pp. 485-491.
68) DOI>Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. Friedrich, K., Vieira, F.A., Porrozzi, R., Marchevsky, R.S., Miekeley, N., Grimaldi, G., Paumgartten, F.J.R.2012.Journal of Toxicology and Environmental Health - Part A: Current Issues 75 (2), pp. 63-75.
69) DOI>Evaluation of the organic matter sources using the δ13C composition of individual n-alkanes in sediments from Brazilian estuarine systems by GC/C/IRMS. Maioli, O.L.G., de Oliveira, C.R., Dal Sasso, M.A., Madureira, L.A.S., Azevedo, D.A., de Aquino Neto, F.R. 2012. Estuarine, Coastal and Shelf Science 114, pp. 140-147.
70) DOI>Development and validation of a ultra high performance liquid chromatography-tandem mass spectrometric method for the direct detection of formoterol in human urine. Sardela, V.F., Deventer, K., Pereira, H.M.G., de Aquino Neto, F.R., Van Eenoo, P. 2012. Journal of Pharmaceutical and Biomedical Analysis 70, pp. 471-475.
71) DOI>Determination of six pterins in urine by LC-MS/MS. Allegri, G., Costa Netto, H.J.B., Ferreira Gomes, L.N.L., Costa De Oliveira, M.L., Scalco, F.B., De Aquino Neto, F.R. 2012. Bioanalysis 4 (14), pp. 1739-1746.
72) DOI>Mechanisms associated to impaired activity of cardiac P-type ATPases in endothelial nitric oxide synthase knockout mice. Rezende, D.C., Pôças, E.S.C., Muzi-Filho, H., Cunha, V.M.N., Caricati-Neto, A., Jurkiewicz, A., Noël, F., Quintas, L.E.M. 2012. Journal of Physiology and Biochemistry, pp. 1-8. Article in Press.
73) DOI>Natriuretic effect of bufalin in isolated rat kidneys involves activation of the Na +-K +-ATPase-Src kinase pathway. Arnaud-Batista, F.J., Costa, G.T., de Oliveira, I.M.B., Costa, P.P.C., Santos, C.F., Fonteles, M.C., Uchôa, D.E., Silveira, E.R., Cardi, B.A., Carvalho, K.M., Amaral, L.S., Pôças, E.S.C., Quintas, L.E.M., Noël, F., Nascimento, N.R.F. 2012. American Journal of Physiology - Renal Physiology 302 (8), pp. F959-F966.
74) DOI>Adipose-derived stem-cell treatment of skeletal muscle injury. Peçanha, R., Bagno, L.D.L.E.S., Ribeiro, M.B., Ferreira, A.B.R., Moraes, M.O., Zapata-Sudo, G., Kasai-Brunswick, T.H., Campos-de-Carvalho, A.C., Goldenberg, R.C.S., Werneck-de-Castro, J.P.S. 2012.Journal of Bone and Joint Surgery - Series A 94 (7), pp. 609-617.
75) DOI>Gallium complexes as new promising metallodrug candidates. Lessa, J.A., Parrilha, G.L., Beraldo, H. 2012. Inorganica Chimica Acta 393, pp. 53-63.
76) DOI>Coordination of thiosemicarbazones and bis(thiosemicarbazones) to bismuth(III) as a strategy for the design of metal-based antibacterial agents. Lessa, J.A., Reis, D.C., Da Silva, J.G., Paradizzi, L.T., Da Silva, N.F., De Fátima A. Carvalho, M., Siqueira, S.A., Beraldo, H. 2012. Chemistry and Biodiversity 9 (9), pp. 1955-1966.
77) DOI>Investigation on the pharmacological profile of 2,6-diacetylpyridine bis(benzoylhydrazone) derivatives and their antimony(III) and bismuth(III) complexes. Ferraz, K.S.O., Silva, N.F., Da Silva, J.G., De Miranda, L.F., Romeiro, C.F.D., Souza-Fagundes, E.M., Mendes, I.C., Beraldo, H. 2012. European Journal of Medicinal Chemistry 53, pp. 98-106.
78) DOI>Spectroscopic and electrochemical characterization of gold(I) and gold(III) complexes with glyoxaldehyde bis(thiosemicarbazones): Cytotoxicity against human tumor cell lines and inhibition of thioredoxin reductase activity. Lessa, J.A., Ferraz, K.S.O., Guerra, J.C., De Miranda, L.F., Romeiro, C.F.D., Souza-Fagundes, E.M., Barbeira, P.J.S., Beraldo, H. 2012. BioMetals 25 (3), pp. 587-598.
79) DOI>N 4-Phenyl-substituted 2-acetylpyridine thiosemicarbazones: Cytotoxicity against human tumor cells, structure-activity relationship studies and investigation on the mechanism of action. Soares, M.A., Lessa, J.A., Mendes, I.C., Da Silva, J.G., Dos Santos, R.G., Salum, L.B., Daghestani, H., Andricopulo, A.D., Day, B.W., Vogt, A., Pesquero, J.L., Rocha, W.R., Beraldo, H. 2012. Bioorganic and Medicinal Chemistry 20 (11), pp. 3396-3409.
80) DOI>Complexation of 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones to copper(II) as an effective strategy for antimicrobial activity improvement. Recio Despaigne, A.A., Da Costa, F.B., Piro, O.E., Castellano, E.E., Louro, S.R.W., Beraldo, H. 2012. Polyhedron 38 (1), pp. 285-290.
81) DOI>2-Acetylpyridine- and 2-benzoylpyridine-derived hydrazones and their gallium(III) complexes are highly cytotoxic to glioma cells. Despaigne, A.A.R., Parrilha, G.L., Izidoro, J.B., Da Costa, P.R., Dos Santos, R.G., Piro, O.E., Castellano, E.E., Rocha, W.R., Beraldo, H. 2012. European Journal of Medicinal Chemistry 50, pp. 163-172.
82) DOI>Influence of susceptibility to hydrolysis and hydrophobicity of arylsemicarbazones on their anti-nociceptive and anti-inflammatory activities. Vieira, R.P., Lessa, J.A., Ferreira, W.C., Costa, F.B., Bastos, L.F.S., Rocha, W.R., Coelho, M.M., Beraldo, H. 2012. European Journal of Medicinal Chemistry 50, pp. 140-148.
83) DOI>Coordination of lapachol to bismuth(III) improves its anti-inflammatory and anti-angiogenic activities. Parrilha, G.L., Vieira, R.P., Campos, P.P., Silva, G.D.F., Duarte, L.P., Andrade, S.P., Beraldo, H. 2012. BioMetals 25 (1), pp. 55-62.
84) DOI>Structural studies on acetophenone- and benzophenone-derived thiosemicarbazones and their zinc(II) complexes. Ferraz, K.S.O., Silva, N.F., Da Silva, J.G., Speziali, N.L., Mendes, I.C., Beraldo, H. 2012. Journal of Molecular Structure 1008, pp. 102-107.
85) DOI>2-Acetylpyridine- and 2-benzoylpyridine-derived thiosemicarbazones and their antimony(III) complexes exhibit high anti-trypanosomal activity. Parrilha, G.L., Dias, R.P., Rocha, W.R., Mendes, I.C., Benítez, D., Varela, J., Cerecetto, H., González, M., Melo, C.M.L., Neves, J.K.A.L., Pereira, V.R.A., Beraldo, H. 2012.Polyhedron 31 (1), pp. 614-621.
2012. Journal of the Brazilian Chemical Society 23 (6), pp. 1054-1061.
86) PubMedMineral content is related to antioxidant and antimutagenic properties of grape juice.Dani, C., Oliboni, L.S., Pra, D., Bonatto, D., Santos, C.E., Yoneama, M.L., Dias, J.F., Salvador, M., Henriques, J.A. 2012. Genetics and molecular research: GMR 11 (3), pp. 3154-3163.
87) DOI>Relationships between chromatin remodeling and DNA damage repair induced by 8-methoxypsoralen and UVA in yeast Saccharomyces cerevisiae. Cruz, L.A., Guecheva, T.N., Bonato, D., Henriques, J.A.P. 2012. Genetics and Molecular Biology 35 (4 SUPPL.), pp. 1052-1059.
88) DOI>Susceptibility to DNA damage in workers occupationally exposed to pesticides, to tannery chemicals and to coal dust during mining. Kvitko, K., Bandinelli, E., Henriques, J.A.P., Heuser, V.D., Rohr, P., da Silva, F.R., Schneider, N.B., Fernandes, S., Ancines, C., da Silva, J. 2012. Genetics and Molecular Biology 35 (4 SUPPL.), pp. 1060-1068.
89)DOI>DNA damage in organs of mice treated acutely with patulin, a known mycotoxin. de Melo, F.T., de Oliveira, I.M., Greggio, S., Dacosta, J.C., Guecheva, T.N., Saffi, J., Henriques, J.A.P., Rosa, R.M. 2012. Food and Chemical Toxicology 50 (10), pp. 3548-3555.
90) DOI>Saccharomyces cerevisiae as a model system to study the response to anticancer agents. Matuo, R., Sousa, F.G., Soares, D.G., Bonatto, D., Saffi, J., Escargueil, A.E., Larsen, A.K., Henriques, J.A.P. 2012. Cancer Chemotherapy and Pharmacology 70 (4), pp. 491-502.
91) DOI>PARPs and the DNA damage response. Sousa, F.G., Matuo, R., Soares, D.G., Escargueil, A.E., Henriques, J.A.P., Larsen, A.K., Saffi, J. 2012. Carcinogenesis 33 (8), pp. 1433-1440.
92) DOI>Neuroprotective and anticonvulsant effects of organic and conventional purple grape juices on seizures in Wistar rats induced by pentylenetetrazole. Rodrigues, A.D., Scheffel, T.B., Scola, G., Santos, M.T.D., Fank, B., De Freitas, S.C.V., Dani, C., Vanderlinde, R., Henriques, J.A.P., Coitinho, A.S., Salvador, M. 2012. Neurochemistry International 60 (8), pp. 799-805.
93) DOI>Re: The best sampling time in buccal micronucleus cytome assay. Cassini, C., Calloni, C., Bortolini, G., Garcia, S.C., Dornelles, M.A., Henriques, J.A.P., Erdtmann, B., Salvador, M. 2012. International Journal of Occupational Medicine and Environmental Health 25 (3), pp. 314-315.
94) DOI>Bioguided fractionation shows Cassia alata Extract to inhibit Staphylococcus epidermidis and Pseudomonas aeruginosa growth and biofilm formation. Saito, S.T., Trentin, D.D.S., MacEdo, A.J., Pungartnik, C., Gosmann, G., Silveira, J.D.D., Guecheva, T.N., Henriques, J.A.P., Brendel, M.2012. Evidence-based Complementary and Alternative Medicine 2012, art.no. 867103.
95) DOI>Iron and genome stability: An update. Prá, D., Franke, S.I.R., Henriques, J.A.P., Fenech, M. 2012. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis 733 (1-2), pp. 92-99.
96) DOI>DNA-damage effect of polycyclic aromatic hydrocarbons from urban area, evaluated in lung fibroblast cultures. Teixeira, E.C., Pra, D., Idalgo, D., Henriques, J.A.P., Wiegand, F. 2012. Environmental Pollution 162, pp. 430-438.
97) DOI>Enhanced resistance of yeast mutants deficient in low-affinity iron and zinc transporters to stannous-induced toxicity. Viau, C.M., Cardone, J.M., Guecheva, T.N., Yoneama, M.-L., Dias, J.F., Pungartnik, C., Brendel, M., Saffi, J., Henriques, J.A.P. 2012.Chemosphere 86 (5), pp. 477-484.
98) DOI>Both XPA and DNA polymerase eta are necessary for the repair of doxorubicin-induced DNA lesions. Moraes, M.C.S., de Andrade, A.Q., Carvalho, H., Guecheva, T., Agnoletto, M.H., Henriques, J.A.P., Sarasin, A., Stary, A., Saffi, J., Menck, C.F.M. 2012. Cancer Letters 314 (1), pp. 108-118.
99) DOI>Improving differential evolution accuracy for flexible ligand docking using a multi-solution strategy. De Magalhães, C.S., Dos S. Barbosa, C.H., Almeida, D.M., Dardenne, L.E. 2012. Lecture Notes in Computer Science. 7435 LNCS, pp. 688-698.
100) DOI>Vasodilatory activity and antihypertensive profile mediated by inhibition of phosphodiesterase type 1 induced by a novel sulfonamide compound. Pontes, L.B., Antunes, F., Sudo, R.T., Raimundo, J.M., Lima, L.M., Barreiro, E.J., Zapata-Sudo, G. 2012. Fundamental and Clinical Pharmacology 26 (6), pp. 690-700.
101) DOI>Synthesis and pharmacological evaluation of novel phenyl sulfonamide derivatives designed as modulators of pulmonary inflammatory response. De Castro Barbosa, M.L., Ramos, T.J.F., De Arantes, A.C.S., Martins, M.A., Silva, P.M.R., Barreiro, E.J., Lima, L.M. 2012. Molecules 17 (12), pp. 14651-14672.
102) DOI>Design, synthesis, antinociceptive and anti-inflammatory activities of novel piroxicam analogues.De Miranda, A.S., Bispo Jr., W., Da Silva, Y.K.C., Magna Suzana Alexandre-Moreira, De Paula Castro, R., Sabino, J.R., Lião, L.M., Lima, L.M.,, Barreiro, E.J. 2012. Molecules 17 (12), pp. 14126-14145.
103) DOI>Design, synthesis, and pharmacological evaluation of novel hybrid compounds to treat sickle cell disease symptoms.Part II: Furoxan derivatives. Dos Santos, J.L., Lanaro, C., Chelucci, R.C., Gambero, S., Bosquesi, P.L., Reis, J.S., Lima, L.M., Cerecetto, H., González, M., Costa, F.F., Chung, M.C. 2012. Journal of Medicinal Chemistry 55 (17), pp. 7583-7592.
104) DOI>Benzenesulfonamide attenuates monocrotaline-induced pulmonary arterial hypertension in a rat model. Zapata-Sudo, G., Pontes, L.B., Silva, J.S.D., Lima, L.M., Nunes, I.K.D.C., Barreiro, E.J., Sudo, R.T. 2012. European Journal of Pharmacology 690 (1-3), pp. 176-182.
105)DOI>LASSBio-542: Novel thalidomide analog distinctly modulates IL-10 and inhibits angiogenesis. de Carvalho, D.S., Lima, L.M., de Lacerda Barreiro, E.J., Alves, T.R., da Costa Nery, J.A., Sarno, E.N., Sampaio, E.P. 2012. Current Bioactive Compounds 8 (2), pp. 167-175.
106) DOI>Synthesis, Biological Evaluation, and Structure-activity Relationship of Clonazepam, Meclonazepam, and 1,4-Benzodiazepine Compounds with Schistosomicidal Activity. Menezes, C.M.S., Rivera, G., Alves, M.A., Do Amaral, D.N., Thibaut, J.P.B., Noël, F., Barreiro, E.J., Lima, L.M. 2012. Chemical Biology and Drug Design 79 (6), pp. 943-949.
107) DOI>Discovery of new orally effective analgesic and anti-inflammatory hybrid furoxanyl N-acylhydrazone derivatives.Hernández, P., Cabrera, M., Lavaggi, M.L., Celano, L., Tiscornia, I., Rodrigues Da Costa, T., Thomson, L., Bollati-Fogolín, M., Miranda, A.L.P., Lima, L.M., Barreiro, E.J., González, M., Cerecetto, H. 2012. Bioorganic and Medicinal Chemistry 20 (6), pp. 2158-2171.
108) DOI>1H HRMAS NMR spectroscopy and chemometrics for evaluation of metabolic changes in Citrus sinensis caused by Xanthomonas axonopodis pv. Citri. Silva, L.M.A., Alves Filho, E.G., Choze, R., Lião, L.M., Alcantara, G.B.
109) DOI>The use of dyed bacterial cellulose to monitor cellulase complex activity. Tiboni, M., Grzybowski, A., Passos, M., Barison, A., Lião, L.M., Campos, F.R., Pontarolo, R., Fontana, J.D. 2012. Cellulose 19 (6), pp. 1867-1877.
110) DOI>Discrimination of biodiesel blends with 1H NMR spectroscopy and principal component analyses. Flores, I.S., Godinho, M.S., De Oliveira, A.E., Alcantara, G.B., Monteiro, M.R., Menezes, S.M.C., Lião, L.M. 2012. Fuel 99, pp. 40-44.
111) DOI>Microbial β-glycosylation of entacapone by Cunninghamella echinulata ATCC 9245.Lustosa, K.R.M.D., Menegatti, R., Braga, R.C., Lião, L.M., De Oliveira, V. 2012. Journal of Bioscience and Bioengineering 113 (5), pp. 611-613.
112) DOI>Absolute configuration of strictosidinic acid.Castro, R.D.P., Matos, C.D.S., Nascimento, C.A.D., Oliveira, C.M.A., Kato, L., Lião, L.M., Sabino, J.R. 2012. Acta Crystallographica Section C: Crystal Structure Communications 68 (4), pp. m94-m96.
113) DOI>Synthesis of the C1-C9 fragment of the potent antitumor agent dictyostatin. Dias, L.C., Sant'ana, D.P., Vieira, Y.W., Gonçalves, C.C.S., Lima, D.J.P. 2012. Journal of the Brazilian Chemical Society 23 (2), pp. 344-348.
114) DOI>Total synthesis of (-)-goniotrionin. Dias, L.C., Ferreira, M.A.B.2012.Journal of Organic Chemistry 77 (8), pp. 4046-4062.
115) DOI>1,5-stereoinduction in boron-mediated aldol reactions of β,δ-bisalkoxy methylketones containing cyclic protecting groups. Dias, L.C., Polo, E.C., Ferreira, M.A.B., Tormena, C.F. 2012. Journal of Organic Chemistry 77 (8), pp. 3766-3792.
116) DOI>The role of β-bulky substituents in aldol reactions of boron enolates of methylketones with aldehydes: Experimental and theoretical studies by DFT analysis. Dias, L.C., De Lucca, E.C., Ferreira, M.A.B., Garcia, D.C., Tormena, C.F. 2012. Journal of Organic Chemistry 77 (4), pp. 1765-1788.
117) DOI>Stereoselective synthesis of analogs of the macrolactone of isomigrastatin.Dias, L.C., Monteiro, G.C., Amarante, G.W., Conegero, L.S., Finelli, F.G. 2012. Tetrahedron Letters 53 (6), pp. 707-709.
118) DOI>Antinociceptive effects of an extract, fraction and an isolated compound of the stem bark of maytenus rigida. Martins, M.V., Estevam, C.S., Santos, A.L.L.M., Dias, A.S., Cupertino-da-Silva, Y.K., Araújo-Júnior, J.X., Miranda, A.L.P., Barreiro, E.J., Pizza, C., Piacente, S., Montoro, P., Quintans-Júnior, L.J., Araujo, B.S., Alexandre-Moreira, M.S., Sant'Ana, A.E.G. 2012. Brazilian Journal of Pharmacognosy 22 (3), pp. 598-603.
119) DOI>Cytotoxic cordiaquinones from the roots of Cordia polycephala.Freitas, H.P.S., Maia, A.I.V., Silveira, E.R., Marinho Filho, J.D.B., Moraes, M.O., Pessoa, C., Costa Lotufo, L.V., Pessoa, O.D.L. 2012. Journal of the Brazilian Chemical Society 23 (8), pp. 1558-1562.
120) DOI>Participation of the NO/cGMP/K+ATP pathway in the antinociception induced by Walker tumor bearing in rats. Barbosa, A.L.R., Pinheiro, C.A., Oliveira, G.J., Torres, J.N.L., Moraes, M.O., Ribeiro, R.A., Vale, M.L., Souza, M.H.L.P. 2012. Brazilian Journal of Medical and Biological Research 45 (6), pp. 531-536.
121) DOI>Evaluating methods for the isolation of marine-derived fungal strains and production of bioactive secondary metabolites. Kossuga, M.H., Romminger, S., Xavier, C., Milanetto, M.C., do Valle, M.Z., Pimenta, E.F., Morais, R.P., Carvalho, E., Mizuno, C.M., Coradello, L.F.C., Barroso, V. M., Vacondio, B., Javaroti, D.C.D., Seleghim, M.H.R., Cavalcanti, B.C., Pessoa, C., Moraes, M.O., Lima, B.A., Gonçalves, R., Bonugli-Santos, R.C., Sette, L.D., Berlinck, R.G.S. 2012. Brazilian Journal of Pharmacognosy 22 (2), pp. 257-267.
122) DOI>Polymorphism evaluation in generic tablets containing mebendazole by dissolution tests. Honorato, S.B., Farfan, S., Viana, A., Filho, J.M., Camarão, G.C., Fechine, F.V., Moraes, M.E.A., Moraes, M.O., Ferro, M., Dabbene, V., Cuffini, S.L., Ayala, A.P. 2012. Journal of the Brazilian Chemical Society 23 (2), pp. 220-227.
123) DOI>Chronic toxicologic study of the ethanolic extract of the aerial parts of Jatropha gossypiifolia in rats.Mariz, S.R., Cerqueira, G.S., Araújo, W.C., Dantas, J.G., Ramalho, J.A., Palomaro, T.V., Duarte, J.C., dos Santos, H.B., Olveira, K., de Araújo, M.S.T., Diniz, M.F.F.M., de Medeiros, I.A. 2012. Brazilian Journal of Pharmacognosy 22 (3), pp. 663-668.
124) DOI>Antiproliferative activity of Eremanthus crotonoides extracts and centratherin demonstrated in brain tumor cell lines. Lobo, J.F.R., Castro, E.S., Gouvea, D.R., Fernandes, C.P., de Almeida, F.B., de Amorim, L.M.F., Burth, P., Rocha, L., Santos, M.G.,Harmerski, L., Lopes, N.P., Pinto, A.C. 2012. Brazilian Journal of Pharmacognosy 22 (6), pp. 1295-1300.
125) LinkCytotoxic and antitumor activities of Hyptis pectinata (Sambacaitá) extract.Barbosa. C.V., Aquino, P.G.V., Ribeiro-Júnior, K.A.L., Moura, F.B.P., Alexandre-Moreira, M.S., Sant'Ana, A.E.G., Ferreira, J.R.O., Moraes, M. O., Pessoa, C., Aguiar, J.S., Silva, T.G., Araújo-Júnior, J.X. 2012. Pharmacologyonline 3, pp. 70-74.
126) DOI>Antinociceptive and anti-inflammatory activities of crude methanolic extract of red alga Bryothamnion triquetrum.Cavalcante-Silva, L.H.A., Da Matta, C.B.B., De Araújo, M.V., Barbosa-Filho, J.M., De Lira, D.P., De Oliveira Santos, B.V., De Miranda, G.E.C., Alexandre-Moreira, M.S. 2012. Marine Drugs 10 (9), pp. 1977-1992.
127) DOI>1-(7-Chloroquinolin-4-yl)-2-[(1H-pyrrol-2-yl)methylene] hydrazine: A potent compound against cancer. Montenegro, R.C., Lotufo, L.V., De Moraes, M.O., Pessoa, C.D., Rodrigues, F.A.R., De Lima Ferreira Bispo, M., De Alcantara, C.C., Kaiser, C.R., De Souza, M.V.N. 2012. Medicinal Chemistry Research 21 (11), pp. 3615-3619.
128) DOI>TP53 mutations in astrocytic gliomas: An association with histological grade, TP53 codon 72 polymorphism and p53 expression. Faria, M.H.G., Neves Filho, E.H.C., Alves, M.K.S., Burbano, R.M.R., de Moraes Filho, M.O., Rabenhorst, S.H.B. 2012. APMIS 120 (11), pp. 882-889.
129) DOI>Genetic toxicology evaluation of essential oil of Alpinia zerumbet and its chemoprotective effects against H2O 2-induced DNA damage in cultured human leukocytes. Cavalcanti, B.C., Ferreira, J.R.O., Cabral, I.O., Magalhães, H.I.F., de Oliveira, C.C., Rodrigues, F.A.R., Rocha, D.D., Barros, F.W.A., Silva, C.R., Júnior, H.V.N., Canuto, K.M., Silveira, E.R., Pessoa, C., Moraes, M.O. 2012. Food and Chemical Toxicology 50 (11), pp. 4051-4061.
130) PubMed Mangiferin ameliorates 6-hydroxydopamine induced cytotoxicity and oxidative stress in ketamine model of schizophrenia. Rao, V.S., Carvalho, A.C., Trevisan, M.T.S., Andrade, G.M., Nobre Jr., H.V., Moraes, M.O., Iury, H.I.M.H., Morais, T.C., Santos, F.A. 2012. Pharmacological Reports 64 (4), pp. 848-856.
131) DOI>Antiproliferative effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in human leukemia cell lines.Faheina-Martins, G.V., da Silveira, A.L., Cavalcanti, B.C., Ramos, M.V., Moraes, M.O., Pessoa, C., Araújo, D.A.M. 2012. Toxicology in Vitro 26 (7), pp. 1161-1169.
132)DOI>Synthesis and in vitro anticancer activity of novel thiazacridine derivatives. da Rocha Pitta, M.G., Souza, E.S., Barros, F.W.A., Moraes Filho, M.O., Pessoa, C.O., Hernandes, M.Z., do Carmo Alves de Lima, M., Galdino, S.L., da Rocha Pitta, I. 2012. Medicinal Chemistry Research, pp. 1-9. Article in Press.
133) DOI>Involvement of intrinsic mitochondrial pathway in neosergeolide-induced apoptosis of human HL-60 leukemia cells: The role of mitochondrial permeability transition pore and DNA damage. Cavalcanti, B.C., Da Costa, P.M., Carvalho, A.A., Rodrigues, F.A.R., Amorim, R.C.N., Silva, E.C.C., Pohlit, A.M., Costa-Lotufo, L.V., Moraes, M.O., Pessoa, C. 2012. Pharmaceutical Biology 50 (8), pp. 980-993.
134) DOI>Efficacy of the Mentha crispa in the treatment of women with Trichomonas vaginalis infection.Moraes, M.E.A., Cunha, G.H., Bezerra, M.M., Fechine, F.V., Pontes, A.V., Andrade, W.S., Bezerra, F.A.F., Moraes, M.O., Cavalcanti, P.P.2012. Archives of Gynecology and Obstetrics 286 (1), pp. 125-130.
135) DOI>Global pharmacogenomics: Impact of population diversity on the distribution of polymorphisms in the CYP2C cluster among Brazilians. Suarez-Kurtz, G., Genro, J.P., De Moraes, M.O., Ojopi, E.B., Pena, S.D.J., Perini, J.A., Ribeiro-Dos-Santos, A., Romano-Silva, M.A., Santana, I., Struchiner, C.J. 2012. Pharmacogenomics Journal 12 (3), pp. 267-276.
136) DOI>Influence of Genomic Ancestry on the Distribution of SLCO1B1, SLCO1B3 and ABCB1 Gene Polymorphisms among Brazilians.Sortica, V.A., Ojopi, E.B., Genro, J.P., Callegari-Jacques, S., Ribeiro-dos-Santos, A., de Moraes, M.O., Romano-Silva, M.A., Pena, S.D.J., Suarez-Kurtz, G., Hutz, M.H. 2012. Basic and Clinical Pharmacology and Toxicology 110 (5), pp. 460-468.
137) DOI>Polysaccharide isolated from Passiflora edulis: Characterization and antitumor properties. Silva, D.C., Freitas, A.L.P., Barros, F.C.N., Lins, K.O.A.L., Alves, A.P.N.N., Alencar, N.M.N., De Figueiredo, I.S.T., Pessoa, C., Moraes, M.O., Costa-Lotufo, L.V.,Feitosa, J.P.A., Maciel, J.S., De Paula, R.C.M. 2012. Carbohydrate Polymers 87 (1), pp. 139-145.
138) DOI>Mangiferin binding to serum albumin is non-saturable and induces conformational changes at high concentrations. Freitas, P.G., Barbosa, A.F., Saraiva, L.A., Camps, I., Da Silveira, N.J.F., Veloso, M.P., Santos, M.H., Schneedorf, J.M. 2012. Journal of Luminescence 132 (11), pp. 3027-3034.
139) DOI>Effectiveness of Cissampelos sympodialis and its isolated alkaloid warifteine in airway hyperreactivity and lung remodeling in a mouse model of asthma.Bezerra-Santos, C.R., Vieira-De-Abreu, A., Vieira, G.C., Filho, J.R., Barbosa-Filho, J.M., Pires, A.L., Martins, M.A., Souza, H.S.,Bandeira-Melo, C., Bozza, P.T., Piuvezam, M.R. 2012. International Immunopharmacology 13 (2), pp. 148-155.
140)DOI>Immunosuppressants: Implications in Orthodontics. dos Santos, R.L., Lacerda, M.C.M., Gonçalves, R.T., Martins, M.A., de Souza, M.M.G. 2012. Dental Press Journal of Orthodontics 17 (2), pp. 55-61.
141) DOI>Anti-nociceptive activity of Pereskia bleo Kunth. (Cactaceae) leaves extracts. Abdul-Wahab, I.R., Guilhon, C.C., Fernandes, P.D., Boylan, F.2012.Journal of Ethnopharmacology 144 (3), pp. 741-746.
142) DOI>Antinociceptive effect of the Orbignya speciosa Mart. (Babassu) leaves: Evidence for the involvement of apigenin.Pinheiro, M.M.G., Boylan, F., Fernandes, P.D. 2012.Life Sciences 91 (9-10), pp. 293-300.
143) DOI>Pharmacological mechanisms involved in the antinociceptive effects of dexmedetomidine in mice.Rangel, R.A.S., Marinho, B.G., Fernandes, P.D., de Moura, R.S., Lessa, M.A. 2012. Fundamental and Clinical Pharmacology.Article in Press.
144) DOI>A novel toxic alkaloid from poison hemlock (Conium maculatum L., Apiaceae): Identification, synthesis and antinociceptive activity. Radulović, N., DorDević, N., Denić, M., Pinheiro, M.M.G., Fernandes, P.D., Boylan, F. 2012. Food and Chemical Toxicology 50 (2), pp. 274-279.
145) DOI>Year in review in Intensive Care Medicine 2012: I. Neurology and neurointensive care, epidemiology and nephrology, biomarkers and inflammation, nutrition, experimental. Antonelli, M., Bonten, M., Cecconi, M., Chastre, J., Citerio, G., Conti, G., Curtis, J.R., Hedenstierna, G., Joannidis, M., Macrae, D., Maggiore, S.M., Mancebo, J., Mebazaa, A., Preiser, J-C., Rocco, P., Timsit, J-F., Wernerman, J., Zhang, H. 2012. Intensive Care Medicine , pp. 1-15.Article in Press.
146) PubMedMechanical ventilation in obese patients.Leme Silva, P., Pelosi, P., Rocco, P.R.M. 2012. Minerva Anestesiologica 78 (10), pp. 1136-1145.
129) DOI>Ventilator-induced lung injury. Maron-Gutierrez, T., Pelosi, P., Rocco, P.R.M. 2012. European Respiratory Monograph 55, pp. 1-18.
147) DOI>Protective effects of bone marrow mononuclear cell therapy on lung and heart in an elastase-induced emphysema model.Cruz, F.F., Antunes, M.A., Abreu, S.C., Fujisaki, L.C., Silva, J.D., Xisto, D.G., Maron-Gutierrez, T., Ornellas, D.S., Sá, V.K., Rocha, N.N., Capelozzi, V.L., Morales, M.M., Rocco, P.R.M.2012. Respiratory Physiology and Neurobiology 182 (1), pp. 26-36.
148) DOI>Intratracheal instillation of lipopolymeric vectors and the effect on mice lung physiology.Xisto, D.G., Temprana, C.F., Martini, S.V., Silva, A.L., Abreu, S.G., Silva, J.D., Crosseti, J., Rocco, P., Alonso, S.V., Morales, M.M. 2012. Cellular Physiology and Biochemistry 29 (5-6), pp. 791-798.
149) DOI>Year in review in Intensive Care Medicine 2011: III. ARDS and ECMO, weaning, mechanical ventilation, noninvasive ventilation, pediatrics and miscellanea.Antonelli, M., Bonten, M., Chastre, J., Citerio, G., Conti, G., Curtis, J.R., De Backer, D., Hedenstierna, G., Joannidis, M., Macrae, D., Mancebo, J., Maggiore, S.M., Mebazaa, A., Preiser, J-C., Rocco, P., Timsit, J-F., Wernerman, J., Zhang, H. 2012. Intensive Care Medicine 38 (4), pp. 542-556.
150) DOI>Pathophysiology of ventilator-associated lung injury.Rocco, P.R.M., Santos, C.D., Pelosid, P. 2012. Current Opinion in Anaesthesiology 25 (2), pp. 123-130.
151) DOI>Regular and moderate exercise before experimental sepsis reduces the risk of lung and distal organ injury. De Araújo, C.C., Silva, J.D., Samary, C.S., Guimarães, I.H., Marques, P.S., Oliveira, G.P., Do Carmo, L.G.R.R., Goldenberg, R.C., Bakker-Abreu, I., Diaz, B.L., Rocha, N.N., Capelozzi, V.L., Pelosi, P., Rocco, P.R.M. 2012. Journal of Applied Physiology 112 (7), pp. 1206-1214.
152) DOI>Effects of pentoxifylline on intestinal bacterial overgrowth, bacterial translocation and spontaneous bacterial peritonitis in cirrhotic rats with ascites.Corradi, F., Brusasco, C., Fernández, J., Vila, J., Ramirez, M.J., Seva-Pereira, T., Fernández-Varo, G., Mosbah, I.B., Acevedo, J., Silva, A., Rocco, P. R. M., Pelosi, P., Gines, P., Navasa, M. 2012. Digestive and Liver Disease 44 (3), pp. 239-244.
153) DOI>Year in review in Intensive Care Medicine 2011. II. Cardiovascular, infections, pneumonia and sepsis, critical care organization and outcome, education, ultrasonography, metabolism and coagulation. Antonelli, M., Bonten, M., Chastre, J., Citerio, G., Conti, G., Curtis, J.R., De Backer, D., Hedenstierna, G., Joannidis, M., Macrae, D., Mancebo, J., Maggiore, S.M., Mebazaa, A., Preiser, J-C., Rocco, P., Timsit, J-F., Wernerman, J., Zhang, H. 2012. Intensive Care Medicine 38 (3), pp. 345-358.
154) DOI>Effects of different tidal volumes in pulmonary and extrapulmonary lung injury with or without intraabdominal hypertension.Santos, C.L., Moraes, L., Santos, R.S., Oliveira, M.G., Silva, J.D., Maron-Gutierrez, T., Ornellas, D.S., Morales, M.M., Capelozzi, V.L., Jamel, N., Pelosi, P., Rocco, P.R.M., Garcia, C.S.N.B. 2012.Intensive Care Medicine 38 (3), pp. 499-508.
155) DOI>Year in review in intensive care medicine 2011: I. Nephrology, epidemiology, nutrition and therapeutics, neurology, ethical and legal issues, experimental. Antonelli, M., Bonten, M., Chastre, J., Citerio, G., Conti, G., Curtis, J.R., De Backer, D., Hedenstierna, G., Joannidis, M., Macrae, D., Mancebo, J., Maggiore, S.M., Mebazaa, A., Preiser, J-C., Rocco, P., Timsit, J-F., Wernerman, J., Zhang, H. 2012. Intensive Care Medicine 38 (2), pp. 192-209.
156) DOI>Central pharmacological activity of a new piperazine derivative: 4-(1-Phenyl-1h-pyrazol-4-ylmethyl)-piperazine-1-carboxylic acid ethyl ester. De Brito, A.F., Martins, J.L.R., Fajemiroye, J.O., Galdino, P.M., De Lima, T.C.M., Menegatti, R., Costa, E.A. 2012. Life Sciences 90 (23-24), pp. 910-916.
157) DOI>Microbial β-glycosylation of entacapone by Cunninghamella echinulata ATCC 9245.Lustosa, K.R.M.D., Menegatti, R., Braga, R.C., Lião, L.M., De Oliveira, V. 2012. Journal of Bioscience and Bioengineering 113 (5), pp. 611-613.
158) DOI>CB1 and CB2 contribute to antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint. Gondim, D.V., Araújo, J.C.B., Cavalcante, A.L.C., Havt, A., Quetz, J.S., de Castro Brito, G.A., Ribeiro, R.A., Vale, M.L. 2012. Canadian Journal of Physiology and Pharmacology 90 (11), pp. 1479-1489.
159) DOI>Protein fraction of Calotropis procera latex protects against 5-fluorouracil-induced oral mucositis associated with downregulation of pivotal pro-inflammatory mediators. Freitas, A.P.F., Bitencourt, F.S., Brito, G.A.C., De Alencar, N.M.N., Ribeiro, R.A., Lima Jr., R.C.P., Ramos, M.V., Vale, M.L. 2012. Naunyn-Schmiedeberg's Archives of Pharmacology 385 (10), pp. 981-990.
160) DOI>Involvement of the NO/cGMP/PKG/KATP pathway and endogenous opioids in the antinociceptive effect of a sulphated-polysaccharide fraction extracted from the red algae, Gracilaria caudate.das Chagas Vieira Júnior, F., Sales, A.B., Barros, F.C.N., Chaves, L.S., Freitas, A.L.P., Vale, M.L., Ribeiro, R.A., Souza, M.H.L.P., Medeiros, J-V.R., Barbosa, A.L.R. 2012. Biomedicine and Preventive Nutrition 2 (4), pp. 303-309.
161) DOI>Glucocorticoid and calcitonin receptor expression in central giant cell lesions: Implications for therapy. Nogueira, R.L.M., Faria, M.H.G., Osterne, R.L.V., Cavalcante, R.B., Ribeiro, R.A., Rabenhorst, S.H.B. 2012. International Journal of Oral and Maxillofacial Surgery 41 (8), pp. 994-1000.
162) DOI>Apolipoprotein E COG 133 mimetic peptide improves 5-fluorouracil-induced intestinal mucositis. Azevedo, O.G.R., Oliveira, R.A.C., Oliveira, B.C., Zaja-Milatovic, S., Araújo, C.V., Wong, D.V.T., Costa, T.B., Lucena, H.B.M., Lima-Júnior, R.C.P., Ribeiro, R.A., Warren, C.A., Lima, A.A.M., Vitek, M.P., Guerrant, R.L., Oriá, R.B.2012. BMC Gastroenterology 12, art.no. 35.
163) DOI>Antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint.Gondim, D.V., Costa, J.L., Rocha, S.S., Brito, G.A.C., Ribeiro, R.A., Vale, M.L. 2012. Canadian Journal of Physiology and Pharmacology 90 (4), pp. 395-405.
164)DOI>Insight into p95HER2 in breast cancer: Molecular mechanisms and targeted therapies. de Mello, R.A., de Vasconcelos, A., Ribeiro, R.A., Pousa, I., Afonso, N., Pereira, D., Rodrigues, H. 2012. Recent Patents on DNA and Gene Sequences 6 (1), pp. 56-63.
165) DOI>An adapted tissue microarray for the development of a matrix arrangement of tissue samples. Gurgel, D.C., Dornelas, C.A., Lima-Júnior, R.C.P., Ribeiro, R.A., Almeida, P.R.C. 2012. Pathology Research and Practice 208 (3), pp. 167-168.
166) DOI>Immunoexpression of cyclooxygenase-2 in primary gastric carcinomas and lymph node metastases.Almeida, P.R.C., Ferreira, F.V.A., Santos, C.C., Rocha-Filho, F.D., Feitosa, R.R.P., Falcão, E.A.A., Cavada, B.K., Lima-Júnior, R.C.P., Ribeiro, R.A. 2012. World Journal of Gastroenterology 18 (8), pp. 778-784.
167) DOI>Cyclooxygenase-2 expression in central giant cell lesion of the jaws: An immunohistochemical study. Nogueira, R.L.M., Faria, M.H.G., Osterne, R.L.V., Cavalcante, R.B., Ribeiro, R.A., Rabenhorst, S.H.B. 2012. Journal of Molecular Histology 43 (1), pp. 59-62.
168)DOI>In-vitro characterization of the pharmacological effects induced by (-)-α-bisabolol in rat smooth muscle preparations. de Siqueira, R.J.B., Freire, W.B.S., Vasconcelos-Silva, A.A., Fonseca-Magalhães, P.A., Lima, F.J.B., Brito, T.S., Mourão, L.T.C., Ribeiro, R.A., Lahlou, S., Magalhães, P.J.C. 2012. Canadian Journal of Physiology and Pharmacology 90 (1), pp. 23-35.
169) DOI>In vivo evaluation of the highly soluble oral β-cyclodextrin- Sertraline supramolecular complexes.Passos, J.J., De Sousa, F.B., Mundim, I.M., Bonfim, R.R., Melo, R., Viana, A.F., Stolz, E.D., Borsoi, M., Rates, S.M.K., Sinisterra, R.D. 2012.International Journal of Pharmaceutics 436 (1-2), pp. 478-485.
170) DOI>Uliginosin B presents antinociceptive effect mediated by dopaminergic and opioid systems in mice. Stolz, E.D., Viana, A.F., Hasse, D.R., von Poser, G.L., do Rego, J.-C., Rates, S.M.K.2012. Progress in Neuro-Psychopharmacology and Biological Psychiatry 39 (1), pp. 80-87.
171) DOI>Acute and repeated-doses (28 days) toxicity study of Hypericum polyanthemum Klotzsch ex Reichardt (Guttiferare) in mice.Betti, A.H., Stein, A.C., Dallegrave, E., Wouters, A.T.B., Watanabe, T.T.N., Driemeier, D., Buffon, A., Rates, S.M.K.2012.Food and Chemical Toxicology 50 (7), pp. 2349-2355.
172) DOI>Acylphloroglucinols from Elaphoglossum crassipes: Antidepressantlike activity of Crassipin A. Socolsky, C., Rates, S.M.K., Stein, A.C., Asakawa, Y., Bardón, A.2012. Journal of Natural Products 75 (6), pp. 1007-1017.
173) DOI>Effect of storage time and conditions on the diene valepotriates content of the extract of Valeriana glechomifolia obtained by supercritical carbon dioxide. Müller, L.G., De Andrade Salles, L., Sakamoto, S., Stein, A.C., Cargnin, S.T., Cassel, E., Vargas, R.F., Rates, S.M.K.,, Von Poser, G.L.2012. Phytochemical Analysis 23 (3), pp. 222-227.
174) DOI>Uliginosin B, a phloroglucinol derivative from Hypericum polyanthemum: A promising new molecular pattern for the development of antidepressant drugs. Stein, A.C., Viana, A.F., Müller, L.G., Nunes, J.M., Stolz, E.D., Do Rego, J.-C., Costentin, J., von Poser, G.L.,, Rates, S.M.K.2012. Behavioural Brain Research 228 (1), pp. 66-73.
175) DOI>Antidepressant-like effect of Valeriana glechomifolia Meyer (Valerianaceae) in mice.Müller, L.G., Salles, L.A., Stein, A.C., Betti, A.H., Sakamoto, S., Cassel, E., Vargas, R.F., von Poser, G.L., Rates, S.M.K.2012.Progress in Neuro-Psychopharmacology and Biological Psychiatry 36 (1), pp. 101-109.
176) PubMedFlavonoids bearing an O-arabinofuranosyl-(1→3)-rhamnoside moiety from Cladocolea micrantha: Inhibitory effect on human melanoma cells.Guimarães, A.C., Magalhães, A., Nakamura, M.J., Siani, A.C., Barja-Fidalgo, C., Sampaio, A.L.F. 2012. Natural Product Communications 7 (10), pp. 1311-1314.
177) DOI>Terpenic fraction of Pterodon pubescens inhibits nuclear factor kappa B and extracellular signal-regulated protein Kinase 1/2 activation and deregulates gene expression in leukemia cells. Pereira, M.F., Martino, T., Dalmau, S.R., Paes, M.C., Barja-Fidalgo, C., Albano, R.M., Coelho, M.G.P., Sabino, K.C.D.C. 2012.BMC Complementary and Alternative Medicine 12, art.no. 231.
178) DOI>Nickel induces apoptosis in human neutrophils. Freitas, M., Barcellos-de-Souza, P., Barja-Fidalgo, C., Fernandes, E. 2012. BioMetals, pp. 1-9.Article in Press.
179) DOI>Vitamin a modulates the expression of genes involved in iron bioavailability. Citelli, M., Bittencourt, L.L., Da Silva, S.V., Pierucci, A.P.T., Pedrosa, C.2012.Biological Trace Element Research 149 (1), pp. 64-70.
180) DOI>Heme modulates smooth muscle cell proliferation and migration via NADPH oxidase: A counter-regulatory role for heme oxygenase system. Moraes, J.A., Barcellos-de-Souza, P., Rodrigues, G., Nascimento-Silva, V., Silva, S.V., Assreuy, J., Arruda, M.A., Barja-Fidalgo, C. 2012. Atherosclerosis 224 (2), pp. 394-400.
181) DOI>Leukotriene B4 inhibits neutrophil apoptosis via NADPH oxidase activity: Redox control of NF-κB pathway and mitochondrial stability. Barcellos-de-Souza, P., Canetti, C., Barja-Fidalgo, C., Arruda, M.A. 2012. Biochimica et Biophysica Acta - Molecular Cell Research 1823 (10), pp. 1990-1997.
182) DOI>A pro-inflammatory profile of endothelial cell in Lonomia obliqua envenomation.Nascimento-Silva, V., Rodrigues da Silva, G., Moraes, J.A., Cyrino, F.Z., Seabra, S.H., Bouskela, E., Guimarães, J.A., Barja-Fidalgo, C. 2012. Toxicon 60 (1), pp. 50-60.
183) DOI> Maxadilan, the Lutzomyia longipalpis vasodilator, drives plasma leakage via PAC1-CXCR1/2-pathway. Svensjö, E., Saraiva, E.M., Amendola, R.S., Barja-Fidalgo, C., Bozza, M.T., Lerner, E.A., Teixeira, M.M., Scharfstein, J. 2012.Microvascular Research 83 (2), pp. 185-193.
184) DOI>Chemopreventive activity of compounds extracted from Casearia sylvestris (Salicaceae) Sw against DNA damage induced by particulate matter emitted by sugarcane burning near Araraquara, Brazil. Prieto, A.M., Santos, A.G., Csipak, A.R., Caliri, C.M., Silva, I.C., Arbex, M.A., Silva, F.S., Bolzani, V.S., Soares, C.P. 2012. Toxicology and Applied Pharmacology 265 (3), pp. 368-372.
185) DOI>Further monoterpene chromane esters from Peperomia obtusifolia: VCD determination of the absolute configuration of a new diastereomeric mixture. Batista Jr., J.M., Batista, A.N.L., Kato, M.J., Bolzani, V.S., López, S.N., Nafie, L.A., Furlan, M. 2012. Tetrahedron Letters 53 (45), pp. 6051-6054.
186) DOI>4'-aminochalcones as novel inhibitors of the chlorinating activity of myeloperoxidase.Zeraik, M.L., Ximenes, V.F., Regasini, L.O., Dutra, L.A., Silva, D.H.S., Fonseca, L.M., Coelho, D., Machado, S.A.S.,, Bolzani, V.S. 2012. Current Medicinal Chemistry 19 (31), pp. 5405-5413.
187) DOI>Application of MALDI-MS analysis of Rainforest chemodiversity: A keystone for biodiversity conservation and sustainable use. Pavarini, D.P., Da Silva, D.B., Carollo, C.A., Portella, A.P.F., Latansio-Aidar, S.R., Cavalin, P.O., Oliveira, V.C., (...), Joly, C.A. 2012. Journal of Mass Spectrometry 47 (11), pp. 1482-1485.
188) DOI>Alkyl hydroxybenzoic acid derivatives that inhibit HIV-1 protease dimerization.Flausino Jr., O.A., Dufau, L., Regasini, L.O., Petrônio, M.S., Silva, D.H.S., Rose, T., Bolzani, V.S., Reboud-Ravaux, M. 2012.Current Medicinal Chemistry 19 (26), pp. 4534-4540.
189) DOI>Antifungal activity of maytenin and pristimerin.Gullo, F.P., Sardi, J.C.O., Santos, V.A.F.F.M., Sangalli-Leite, F., Pitangui, N.S., Rossi, S.A., De Paula E Silva, A.C.A., Soares, L.A., Silva, J.F., Oliveira, H.C., Furlan, M., Silva, D.H.S., Bolzani, V.S., Mendes-Giannini, M.J.S., Fusco-Almeida, A.M. 2012. Evidence-based Complementary and Alternative Medicine 2012, art.no. 340787.
190) DOI>Antiprotozoal sesquiterpene pyridine alkaloids from Maytenus ilicifolia.Santos, V.A.F.F.M., Regasini, L.O., Nogueira, C.R., Passerini, G.D., Martinez, I., Bolzani, V.S., Graminha, M.A.S., Cicarelli, R.M.B.,, Furlan, M. 2012. Journal of Natural Products 75 (5), pp. 991-995.
191) DOI>Gordon M. Cragg, D.Phil., D.Sc. (h.c.): a man for all natural products. Bolzani, V.S., Davies-Coleman, M., Newman, D.J., Singh, S.B. 2012. Journal of natural products 75 (3), pp. 309-310.
192) DOI>Pyridine alkaloids from senna multijuga as acetylcholinesterase inhibitors.Francisco, W., Pivatto, M., Danuello, A., Regasini, L.O., Baccini, L.R., Young, M.C.M., Lopes, N.P., Lopes, J.L.C., Bolzani, V.S. 2012.Journal of Natural Products 75 (3), pp. 408-413.
CONCLUDED MASTER IN SCIENCES (MSc) DISSERTATIONS 2012
- Adriana Lopes da Silva. Impacto do uso de nanopartículas com timulina no remodelamento da via aérea e parênquima em modelo de asma alérgica crônica. 2012. Dissertação (Mestrado em Ciências Biológicas (Fisiologia)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Rieken Macedo Rocco.
- Andressa Braga. Avaliação do efeito de Passiflora alata curtis (Passifloraceae) em modelos animais e ensaios neuroquímicos preditivos de ação sobre o comportamento alimentar e receptor GABA-benzodiazepínico. 2012. Dissertação (Mestrado em Ciências Farmacêuticas) - Universidade Federal do Rio Grande do Sul, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Stela Maris Kuze Rates.
- Andressa Moraes Guimarães da Silva Torres. Desenvolvimento de um modelo de proliferação de fibroblastos pulmonares em sistema de 3D. 2012. Dissertação (Mestrado em Biologia Celular e Molecular) - Fundação Oswaldo Cruz, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Machado Rodrigues e Silva Martins.
- Carlos Frederico Marques Guimarães. Avaliação sistemática da influência de solventes de extração sobre o efeito de matriz na quantificação de cloranfenicol em músculo de frango por cromatografia líquida de alta eficiência acoplada à espectrometria de massas sequencial. 2012. Dissertação (Mestrado em Química) - Universidade Federal do Rio de Janeiro. Orientador: Francisco Radler de Aquino Neto.
- Clara Forrer Charlier. Influência da variabilidade genética sobre biomarcadores de dano oxidativo e não oxidativo em pacientes com doença pulmonar obstrutiva crônica (DPOC). 2012. Dissertação (Mestrado em Biologia Celular e Molecular) - Universidade Federal do Rio Grande do Sul, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Joao Antonio Pegas Henriques.
- Cláudia B. Jacques Foss de Oliveira. Análise do setor de energias renováveis utilizando a prospecção tecnológica. 2012. Dissertação (Mestrado em Tecnologia de processos químicos e bioquímicos) - Escola de Química - UFRJ, Orientador: Adelaide Maria de Souza Antunes.
- Cláudia de Souza Pedrosa. Avaliação da influência de polimorfismos gênicos em voluntários de um estudo de bioequivalência entre duas formulações de varfarina. 2012. Dissertação (Mestrado em Inovação Biofarmacêutica) - Universidade Federal de Minas Gerais, Orientador: Carlos Alberto Tagliati.
- Cristiano Trindade. Avaliação do potencial antigenotóxico e antimutagênico do ditelureto de difenila em fibroblastos de pulmão de hamster chinês V79.. 2012. Dissertação (Mestrado em Biologia Celular e Molecular) - Universidade Federal do Rio Grande do Sul, Orientador: Joao Antonio Pegas Henriques.
- Daniel Nascimento do Amaral. Desenho, síntese e avaliação farmacológica de novos análogos do sunitinibe. 2012. Dissertação (Mestrado em Química) - Universidade Federal do Rio de Janeiro, Orientador: Lidia Moreira Lima.
- Daniele Matheus de Souza. Papel regulatório da proteína anexina-1 e de seu derivado peptídeo ac 2-26 no modelo experimental de asma alérgica em camundongos. 2012. Dissertação (Mestrado em Biologia Humana e Experimental) - Universidade do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Machado Rodrigues e Silva Martins.
- Davidson Furtado Dias. Avaliação do pape; do óxido nítrico no comprometimento da função pulmonar e hiper-reatividade das vias aéreas em camundongos estimulados com sílica. 2012. Dissertação (Mestrado em Biologia Humana e Experimental) - Universidade do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Machado Rodrigues e Silva Martins.
- Elane Cristina Silva dos Santos. Avaliação do potencial tóxico do extrato hidroalcoólico de pradosia huberi ducke sobre o Sistema Reprodutor Masculino e Órgãos Vitais de Ratos e sua prole. 2012. Dissertação (Mestrado em Produtos Naturais e Sintéticos Bioativos) - Universidade Federal da Paraíba, Orientador: Margareth de Fátima Formiga Melo Diniz.
- Ethiene Castelluci Estevam. Avaliação da toxicidade pré-clínica do extrato etanólico da casca do caule de zizyphus joazeiro. 2012. Dissertação (Mestrado em Produtos Naturais e Sintéticos Bioativos) - Universidade Federal da Paraíba, Orientador: Margareth de Fátima Formiga Melo Diniz.
- Fabio Junior Moreira Novaes. Aplicações da Cromatografia gasosa de alta temperatura acoplada à espectrometria de massas (CG-AT-EM). 2012. Dissertação (Mestrado em Química) - Universidade Federal do Rio de Janeiro, Orientador: Francisco Radler de Aquino Neto.
- Fernanda Vargas e Silva Castanheira. Mecanismos envolvidos na expressão da GRK2 em neutrófilos: efeito inibitório do sulfeto de hidrogênio. 2012. Dissertação (Mestrado em Farmacologia) - Faculdade de Medicina de Ribeirão Preto - USP, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Fernando de Queiroz Cunha.
- Gildo Barreira Furtado. Avaliação do efeito terapêutico da aroeira do sertão ( myracroduon urundeuva allemão) na gastropatia reativa induzida por anti-inflamatório não esteróides.. 2012. Dissertação (Mestrado em Pós-Graduação em Farmacologia) - Universidade Federal do Ceará, Orientador: Manoel Odorico de Moraes Filho.
- Giovana Vera Bortolini. Atividade antioxidante, antigenotóxica de extratos de folhas de vitis labrusca (Variedade Isabel) Orgânica e Convencional em Células de Mamífero V79. 2012. Dissertação (Mestrado em Biotecnologia) - Universidade de Caxias do Sul, Orientador: João Antonio Pegas Henriques.
- Gisele de Araújo Padilha. Terapia com LASSBio-596 em modelo murino de enfisema pulmonar induzido por elastase.. 2012. Dissertação (Mestrado em Ciências Biológicas (Fisiologia)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Rieken Macedo Rocco.
- Gustavo Claro Monteiro. Síntese e avaliação farmacológica de análogos estruturais da macrolactona da migrastatina. 2012. Dissertação (Mestrado em Química) - Universidade Estadual de Campinas, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Luiz Carlos Dias.
- Isabela Henriques Lucas Guimarães. Impacto do exercício na inflamação e no remodelamento do parênquima pulmonar em modelo de enfisema. 2012. Dissertação (Mestrado em Ciências Biológicas (Fisiologia)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Rieken Macedo Rocco.
- Jonathas Felipe Revoredo Lobo. Investigação fitoquímica e avaliação da atividade antitumoral em glioblastoma multiforme de eremanthus crotonoides (DC.) Sch. Bip. 2012. Dissertação (Mestrado em Química) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Angelo da Cunha Pinto.
- José Henrique Linhares. Avaliação da eficacia terapêutica do xarope de chambá.(justicia pectoralis) em asma.. 2012. Dissertação (Mestrado em Pós-Graduação em cirurgia) - Universidade Federal do Ceará, Orientador: Manoel Odorico de Moraes Filho.
- Katia de Souza Almeida. A estrutura da produção de fontes combustíveis fósseis e biocombustíveis - petróleo & cana-de-açúcar. 2012. Dissertação (Mestrado em Tecnologia de Processos Químicos e Bioquímicos) - Escola de Química - UFRJ, . Orientador: Adelaide Maria de Souza Antunes.
- Lívia Talita Cajaseiras Mourão. Avaliação de mecanismos e mediadores envolvidos no possível efeito anti-inflamatório do pré-condicionamento isquêmico à distância na mucosite intestinal induzida por irinotecano. 2012. Dissertação (Mestrado em Farmacologia) - Universidade Federal do Ceará, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Ronaldo de Albuquerque Ribeiro.
- Marina Amaral Alves. Estudo de novos protótipos de fármacos leismanicidas e/ou tripanomicidas. 2012. Dissertação (Mestrado em Química) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Lidia Moreira Lima.
- Marlon Daniel Lima Tonin. Planejamento, Síntese e avaliação farmacológica de novos derivados N-acilidrazônicos candidatos a fármacos úteis no tratamento da hipertermia maligna. 2012. Dissertação (Mestrado em Ciências Biológicas (Farmacologia e Química Medicinal)) - Universidade Federal do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Carlos Alberto Manssour Fraga.
- Marluce Oliveira Dias. Separação dos Isômeros de alfa/beta-Amirina e Derivados por Cromatografia Líquida de Alta Eficiência - UV. 2012. Dissertação (Mestrado em Química) - Universidade Federal do Rio de Janeiro, Orientador: Angelo da Cunha Pinto.
- Mateus Feitosa Alves. Avaliação psicofarmacológica e toxicológica do R-(+)-limoneno por via inalatória em modelos animais. 2012. Dissertação (Mestrado em Produtos Naturais e Sintéticos Bioativos) - Universidade Federal da Paraíba, Orientador: Margareth de Fátima Formiga Melo Diniz.
- Milena Romeu Gonçalves. Avaliação das atividades antinociceptiva e anti-inflamatória de novos derivados da isatina. 2012. Dissertação (Mestrado em Ciências Biológicas (Farmacologia e Química Medicinal)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Dias Fernandes.
- Monalisa Taveira Brito. Avaliação da atividade antitumoral e toxicidade do óleo essencial das folhas de Rollinia leptopetala R.R. FRIES (Annonaceae) e seus constituintes. 2012. Dissertação (Mestrado em Produtos Naturais e Sintéticos Bioativos) - Universidade Federal da Paraíba, Orientador: Margareth de Fátima Formiga Melo Diniz.
- Patrícia Mendes Jorge. Estudo do mecanismo de ação do Ditelureto de difenila e sua possível interação com ezimas topoisomerase. 2012. Dissertação (Mestrado em Biologia Celular e Molecular) - Universidade Federal do Rio Grande do Sul, Orientador: Joao Antonio Pegas Henriques.
- Rachel Santos Levy. Contribuições aos métodos de análise aplicados na detecção de hormônios peptícos no controle de dopagem. 2012. Dissertação (Mestrado em Bioquímica) - Universidade Federal do Rio de Janeiro, Orientador: Francisco Radler de Aquino Neto.
- Rafael Silveira Amendola. Papel do domínio desintegrina da ADAM-9D na ativação e quimiotaxia de neutrófilos. 2012. Dissertação (Mestrado em Biologia (Biociências Nucleares)) - Universidade do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Thereza Christina Barja Fidalgo.
- Raphael Sanches Peres. Avaliação da função supressora de linfócitos T reguladores (tregs) em pacientes artríticos refratários ao tratamento com metotrexato. 2012. Dissertação (Mestrado em Imunologia Geral e Aplicada) - Faculdade de Medicina de Ribeirão Preto - USP, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Fernando de Queiroz Cunha.
- Thais Emanoelle Tavares Pompeu. Avaliação do potencial mecanismo de ação antipsicótico do LASSBio-579 e de novos derivados N-fenilpiperazínicos e piperazínicos N-substituídos. 2012. Dissertação (Mestrado em Ciências Biológicas (Farmacologia e Química Medicinal)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: François Germain Noël.
- Tiago Fernandes da Silva. Desenho, Síntese e avaliação farmacológicas de novas N-acilidrazonas alifáticas: Análogos Simplificados de LASSBio-294.. 2012. Dissertação (Mestrado em Química) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Eliezer Jesus de Lacerda Barreiro.
- Thiago José Figueira Ramos. Efeito do composto ftalimídico LASSBio-468 sobre a fibrose pulmonar induzida por sílica em camundongos.. 2012. Dissertação (Mestrado em Biologia Celular e Molecular) - Fundação Oswaldo Cruz, Fundação Carlos Chagas Filho de Amparo à Pesq. do Estado do Rio de Janeiro. Orientador: Patricia Machado Rodrigues e Silva Martins.
CONCLUDED DOCTORAL (PhD) THESES 2012
- Alexandre de Andrade Ferreira. Análise isotópica em sistemas petrolíferos da bacia potiguar. 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Orientador: Francisco Radler de Aquino Neto.
- Aline Maria Araújo Martins. Proteômica dos processos inflamatórios crônicos e o estabelecimento do processo neoplásico. 2012. Tese (Doutorado em Biotecnologia - RENORBIO) - Universidade Estadual do Ceará, Orientador: Manoel Odorico de Moraes Filho.
- Ana Cristina Stein. Avaliação do mecanismo de ação antidepressiva e estudo da toxicidade oral aguda e de doses repetidas de hypericum polyanthemum em camundongos. 2012. Tese (Doutorado em Ciências Farmacêuticas) - Universidade Federal do Rio Grande do Sul, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Stela Maris Kuze Rates.
- Ana Paula Bernardo dos Santos. Identificação de substâncias tóxicas de dieffenbachia picta de Mata Atlântica. 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Fundação Carlos Chagas Filho de Amparo à Pesq. do Estado do Rio de Janeiro. Orientador: Angelo da Cunha Pinto.
- Angel Amado Recio Despaigne. Estudo do perfil farmacológico de novos complexos metálicos de hidrazonas derivadas de piridina e imidazóis. 2012. Tese (Doutorado em Química) - Universidade Fedearal de Minas Gerais, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Heloisa de Oliveira Beraldo.
- Carla Cristina Perez. Síntese formal dos policetídeos bicíclicos salinecetais A e B. 2012. Tese (Doutorado em Química) - Universidade Estadual de Campinas, Fundação de Amparo à Pesquisa do Estado de São Paulo. Orientador: Luiz Carlos Dias.
- Carolina Uchoa Guerra Barbosa de Lima. Avaliação da toxicidade aguda e subcrônica e atividade antitumoral do extrato hidroalcoólico bruto das folhas de rollinia leptopetala. 2013. Tese (Doutorado em Produtos Naturais e Sintéticos Bioativos) - Universidade Federal da Paraíba, Orientador: Margareth de Fátima Formiga Melo Diniz.
- Cecília Carvalho de Oliveira. Avaliação da citotoxidade de fármacos em células troncos extraídas do cordão umbilical humano. 2012. Tese (Doutorado em Curso de Pós Graduação Em Farmacologia) - Universidade Federal do Ceará, Orientador: Manoel Odorico de Moraes Filho.
- Cláudio Gleidiston Lima da Silva. "Avaliação da eficácia terapêutica do fluconazol na leishmaniose tegumentar humana". 2012. Tese (Doutorado em Programa de Pós-Graduação em Farmacologia) - Universidade Federal do Ceará, Orientador: Manoel Odorico de Moraes Filho.
- Clélia de Alencar Xavier Mota. Efeito antinociceptivo e anti-inflamatório de 2-(4-nitro-benzilideno)-amino}-4,5,6,7-tetraidro-benzo[b]tiofeno-3-carbonitrila (6CN10) em camundongos. 2012. Tese (Doutorado em Produtos Naturais e Sintéticos Bioativos) - Universidade Federal da Paraíba, Orientador: Margareth de Fátima Formiga Melo Diniz.
- Daniele Carvalho Bernardo Nascimento. Papel das células T reguladoras no desenvolvimento da imunossupressão pós-sepse. 2012. Tese (Doutorado em Imunologia) - Faculdade de Medicina de Ribeirão Preto - USP, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Fernando de Queiroz Cunha.
- Fabrício de Oliveira Souto. Papel dos receptores LXR na sepse. 2012. Tese (Doutorado em Imunologia) - Faculdade de Medicina de Ribeirão Preto - USP, Fundação de Amparo à Pesquisa do Estado de São Paulo. Orientador: Fernando de Queiroz Cunha.
- Fabrício Pires de Moura do Amaral. Efeitos hematológicos e genotóxicos em trabalhadores expostos ocupacionalmente ao cromo, em curtumes do Estado do Piauí. 2012. Tese (Doutorado em Pós Graduação Em Farmacologia) - Universidade Federal do Ceará, Orientador: Manoel Odorico de Moraes Filho.
- Flávio de Almeida Violante. Síntese das Convolutamidinas B e E e estudos sobre as Isatinas. 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Angelo da Cunha Pinto.
- Fernanda Bossemeyer Centurião. Avaliação do efeito de derivados floroglucinóis presentes em hypericum caprifoliatum sobre parâmetros glutamatérgicos e parâmetros relacionados à homeostasia dos íons sódio em roedores.. 2012. Tese (Doutorado em Ciências Farmacêuticas) - Universidade Federal do Rio Grande do Sul, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Stela Maris Kuze Rates.
- Gabriella Allegri Machado. Desenvolvimento de método de análise de pterinas em fluidos biológicos para diagnóstico diferencial de hiperfenilalaninemias. 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Francisco Radler de Aquino Neto.
- Gabrieli Lessa Parrilha. Complexos metálicos de hidrazonas, tiossemicarbazonas e lapachol: atividade farmacológica e avaliação de relações estrutura-atividade. 2012. Tese (Doutorado em Quimica) - Universidade Federal de Minas Gerais, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Heloisa de Oliveira Beraldo.
- Gisele Pena de Oliveira. Efeitos da terapia precoce com glutamina na sepse induzida em ratos desnutridos. 2012. Tese (Doutorado em Ciências Biologicas - Fisiologia) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Rieken Macedo Rocco.
- Hidemburgo Gonçalves Rocha. Isolamento parcial e caracterização da lectinas de leguminosas da região do Cariri com atividade citotóxica inibitória de metástase para células de melanoma B12 transplantadas em camundongos.. 2012. Tese (Doutorado em Curso de Pós Graduação Em Farmacologia) - Universidade Federal do Ceará. Orientador: Manoel Odorico de Moraes Filho.
- Igor Barbosa da Silva. Avaliação do ensaio de potência biológica da alfa-poetina nas linhagens de camundongos B6D2F1, swiss webster, C57Bl/6, NIH e BALB/c. 2012. Tese (Doutorado em Saúde Pública) - Escola Nacional de Saúde Pública Fiocruz, Orientador: Francisco José Roma Paumgartten.
- Josane Alves Lessa. Novos complexos metálicos bioativos com tiossemicarbazonas: investigação do perfil farmacológico e de mecanismos de ação. 2012. Tese (Doutorado em Química) - Universidade Federal de Minas Gerais, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Heloisa de Oliveira Beraldo.
- Karen Rosas Sodré Azevedo. Variação das capacidades vital e inspiratória como parâmetros adicionais para a avaliação da resposta broncodilatadora em pacientes com asma persistente moderada ou grave. 2012. Tese (Doutorado em Clínica Médica) - Universidade Federal do Rio de Janeiro, Orientador: Patricia Rieken Macedo Rocco.
- Karina Oliveira Silva Ferraz. Perfil farmacológico de compostos com aplicações como agentes antineoplásicos, antimicrobianos e contra doenças neuro-degenerativas. 2012. Tese (Doutorado em Química) - Universidade Federal de Minas Gerais, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Heloisa de Oliveira Beraldo.
- Kelvin Stevens Espinola López. Avaliação Farmacológica e Toxicológica dos Extratos Brutos, Frações e Substâncias Isoladas das Folhas de Ottonia anisum Sprengel. 2012. Tese (Doutorado em Ciências Biológicas (Farmacologia e Química Medicinal)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Roberto Takashi Sudo.
- Lavínia Almeida Cruz. Avaliação dos mecanismos de ação da associação de 5-fluorouracil e cisplatina quanto às vias de reparo de dano ao DNA. 2012. Tese (Doutorado em Genética e Biologia Molecular) - Universidade Federal do Rio Grande do Sul, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Joao Antonio Pegas Henriques.
- Marcelo Leite Vieira Costa. Modelo Experimental de esteatohepatite indizida pelo antineoplásico irinotecano: estudo da participação de mediadores inflamatórios (IL-1 e NO) e da translocação bacteriana. 2012. Tese (Doutorado em Farmacologia) - Universidade Federal do Ceará, Orientador: Ronaldo de Albuquerque Ribeiro.
- Márcia Rosa de Almeida. Desenvolvimento e validação de metodologia analítica por CLAE-UV para análise de fitoterápicos, chás e extratos alcoólicos de amostras comerciais de pau-pereira (geissospermum vellosii). 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Angelo da Cunha Pinto.
- Marco Antônio Barbosa Ferreira. Síntese total da (-)-goniotrionina. Estudo teórico da influência estereoeletrônica na seletividade 1,5 em reações aldólicas envolvendo beta-alcoxi metilcetonas. 2012. Tese (Doutorado em Química) - Universidade Estadual de Campinas, Fundação de Amparo à Pesquisa do Estado de São Paulo. Orientador: Luiz Carlos Dias.
- Mariana Martins Gomes Pinheiro. Avaliação antinociceptiva e anti-inflamatória da espécie Choysia ternata Kunth e dos derivados sintéticos N-metilantranilatos de metila, isopropila e propila. 2012. Tese (Doutorado em Programa de Pós-Graduação em Ciências Biológicas (Zoologia)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Dias Fernandes.
- Mariana Renovato Martins. Caracterização da expressão gênica das subpopulações de monócitos CD14+CD16-, CD14+CD16+, CD14dimCD16-. 2012. Tese (Doutorado em Biologia (Biociências Nucleares)) - Universidade do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Thereza Christina Barja Fidalgo.
- Renata Barbosa Lacerda. Novos Derivados N-glicinil-N-acilidrazônicos Heterocíclicos Planejados como Inibidores de MAPK p38. 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Carlos Alberto Manssour Fraga.
- Renata Matuo. Avaliação dos mecanismos de ação do agente antitumoral 5-fluorouracil. 2012. Tese (Doutorado em Biologia Celular e Molecular) - Universidade Federal do Rio Grande do Sul, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Joao Antonio Pegas Henriques.
- Rodolfo do Couto Maia. Novos Derivados N-acilidrazônicos candidatos a protótipos úteis no tratamento da dor crônica inflamatória e neuropática. 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Carlos Alberto Manssour Fraga.
- Samantha Soares Barbosa. Análise de agentes dopantes por cromatografia gasosa bidimensional abrangente acoplada à espectrometria de massas por tempo de vôo (CGxCG-EMTDV). 2012. Tese (Doutorado em Química) - Universidade Federal do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Francisco Radler de Aquino Neto.
- Tatiana Paula Teixeira Ferreira. Estudo do efeito antifibrótico da IL-13PE no controle da resposta pulmonar crônica causada por sílica em camundongos. 2012. Tese (Doutorado em Ciências Biológicas (Farmacologia e Química Medicinal)) - Universidade Federal do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Orientador: Patricia Machado Rodrigues e Silva Martins.
- Thiago Pompermaier Garlet. Papel da IL-10 e IFN- gama na movimentação ortodôntica em camundongos. 2012. Tese (Doutorado em Farmacologia) - Faculdade de Medicina de Ribeirão Preto - USP, Conselho Nacional de Desenvolvimento Científico e Tecnológico. Orientador: Fernando de Queiroz Cunha.
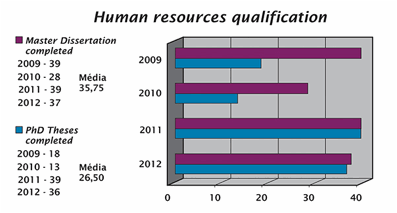
INCT-INOFAR SCHOLARSHIPS
FIOCRUZ/RJ
Julio Beltrame Daleprane CV-Lattes
CNPq Technological Development Scholarship – DTI-3
Time: March 2012 to April 2013
Project: “Study of the potential anti-inflammatory effect of compound LASSBio 897, in models of silicosis and asthma.”
Advisor: Prof. Dr. Marco Aurélio Martins
FIOCRUZ/RJ
Vinicius Frias de Carvalho CV-Lattes
CAPES Pos-doctoral Scholarship
Time: March 2010 to February 2012
Project: “Study of pharmacological interaction of LASSBio-897 and LASSBio-294 with adenosine receptors in living cells.”
Advisor: Prof. Dr. Mraco Aurélio Martins
FIOCRUZ/RJ
UNIFAL
André Victor Pereira CV-Lattes
CNPq Scientific Initiation Scholarship - IC
Time: June 2012 to May 2013
Project: “Technological foresight of intermediaries and synthetic chemical entities of interest in the scope of the INCT-INOFAR.”
Advisor: Prof. Dr. Marcia Paranho Veloso
UNICAMP
Adriano Siqueira Vieira CV-Lattes
CNPq Junior Post-Doctorate Scholarship
Time: August 2009 to July 2012
Project: “Atorvastatin synthesis”
Advisor: Prof. Dr. Luiz Carlos Dias
Institute of Chemistry
Leila de Souza Conegero CV-Lattes
CNPq Junior Post-Doctorate Scholarship
Time: July 2010 to January 2011
Project: “Fluoxetine synthesis”
Advisor: Prof. Dr. Luiz Carlos Dias
Institute of Chemistry
UFC
Bruno Coelho Cavalcanti CV-Lattes
CNPq Junior Post-Doctorate Scholarship
Time: May 2010 to December 2010
Project: “ In vitro evaluation of citotoxic, genotoxic and mutagenic potential of samples provided by INCT-INOFAR.”
Advisor: Prof. Dr. Letícia Veras Costa Lotufo
Unity of Clinical Pharmacology
UFG
Ana Maria Calado Dos Santos CV-Lattes
CNPq Technical Support Scholarship – AT NM
Time: January to June 2011
Project: “In silico prediction and in vitro production of pharmaceutical prototype candidates through bioconversion of human metabolites”
Advisor: Prof. Dr. Valeria de Oliveira
Faculty of Pharmacy
Sarah da Silva Nunes CV-Lattes
CNPq Technical Support Scholarship – AT NM
Time: July 2011 to December 2012
Project: “In silico prediction and in vitro production of pharmaceutical prototype candidates through bioconversion of human metabolites”
Advisor: Prof. Dr. Valeria de Oliveira
Faculty of Pharmacy
UFMG
Carolina Neris Cardoso CV-Lattes
Technological Initiation – ITI A
Time: September 2011 to January 2012
Project: “Semicarbazone Benzaldehyde (BS)”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of Pharmacy
Marcus Vinicius dos Santos CV-Lattes
Technology Undergraduate Grant – ITI A
October 2009 to March 2010
Project: “Benzaldehyde Semicarbazone (BS)”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of Pharmacy
Nathalia Freitas Emiliano CV-Lattes
Technological Initiation – ITI A
Time: September 2011 to January 2012
Project: “Semicarbazone Benzaldehyde (BS)”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of Pharmacy
Samira de Sá e Souza CV-Lattes
Technological Initiation – ITI A
Time: September 2011 to January 2012
Project: “Semicarbazone Benzaldehyde (BS)”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of Pharmacy
Gabrielle Luck de Araujo CV Lattes
CNPq Junior Post-Doctorate Scholarship
Time: July to December 2011
Project: “Semicarbazone Benzaldehyde (BS): toxicological aspects”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of Pharmacy
Wallace Carvalho Ferreira CV-Lattes
CNPQ Technical Support Grant– AT NM
August 2009 to January 2010
Project: “Benzaldehyde Semicarbazone (BS)”
Advisor: Prof. Dr. Marcio de Matos Coelho
Faculty of Pharmacy
UFRJ
Alan Kardec Nogueira de Alencar CV-Lattes
CNPQ Technical Support Grant– AT NM
April to August 2010
Project: Development of new substances for the reduction of ventricular dysfunction, caused by arterial and pulmonary hypertension.
Advisor: Prof. Roberto Takashi Sudo
Institute of Biological Sciences (ICB)
Alan Rodrigues de Sousa CV-Lattes
CNPq Technological Development Scholarship – DTI-3
Time: February 2012 to July 2012
CNPq Technological Suport Scholarship – AT NM
Time: August to 2012 to August 2013
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Alexandra Basilio Lopes CV-Lattes
CNPQ Technological Development Grant– DTI-3
June to September 2010
Project: “Synthesis and evaluation of antinociceptive and anti-inflammatory activities of phenyl-pyridine-n-acylhydrazone compounds planned from imidazo [1,2-a] pyridine-n-acylhydrazone derivatives.”
Advisor: Prof. Eliezer J. Barreiro
LASSBio
Ana Carla Dos Santos CV-Lattes
CNPq Technological Development Scholarship – DTI-3
Time: July 2009 to June 2010
CNPq Technological Development Scholarship – DTI-2
Time: July to 2010 to June 2011
CNPq Technological Development Scholarship – DTI-1
Time: July 2011 to March 2012
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Ana Cristina da Mata Silva CV-Lattes
CNPq Technological Development Scholarship – DTI-3
Time: April 2012 to June 2013
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Arthur Eugen Kümmerle CV-Lattes
Junior Post-Doctoral CNPQ Grant-PDJ
September 2009 to March 2010
Project: “Study of the Inclusion of LASSBio-579 in cyclodextrin.”
Advisor: Prof. Eliezer J. Barreiro
LASSBio
Arthur Henrique Freitas do Prado CV-Lattes
CNPq Technical Support Scholarship – AT NS
Time: May 2011 to February 2012
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Bárbara Assis Novak CV-Lattes
CNPq Scientific Initiation Scholarship - IC
Time: September 2012 to February 2013
Project: “Implementation and validation of pre-clinical trial model for the evaluation of the teratogenic effect of bioactive substances: evaluation of the LASSBio 468 and LASSBio 596 prototypes”
Advisor: Prof. Dr. Aloa Machado de Souza
LASSBio
Carlos Eduardo da Silva Monteiro CV-Lattes
CNPq Technological Development Scholarship – DTI-3
Time: May 2010 to February 2011
Project: “Multitarget activation: strategy for symptomatic treatment of neuropathic pain”
Advisor: Prof. Roberto Takashi Sudo
Institute of Biological Sciences (ICB)
Clemilson Berto Junior CV-Lattes
CAPES Master Scholarship
Time: October 2011 to January 2013
Project: “Evaluation of teratogenic potential of LASSBio 596 and LASSBio 468 prototypes, antiasthma pharmaceutical candidates”
Advisor: Prof. Dr. Aloa Machado
LASSBio
Daniel Nascimento do Amaral CV-Lattes
CAPES Master Scholarship
Time: March 2010 to February 2012
Project: “Design, synthesis and pharmacological evaluation of new antitumor ß –tubulin inhibitor prototypes”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
Fabrício Maia da Silva Salvador CV-Lattes
CNPq Technological Development Scholarship – DTI-3
Time: October 2012 to June 2013
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Givanildo Santos da Silva CV-Lattes
CAPES Doctoral Grant
October 2009 to August 2010
Project: “Studies for the discovery of new anti-influenza, neuramidase inhibitor prototypes.”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
Hannah Carolina Tavares Domingos CV-Lattes
CNPq Scientific Initiation Scholarship - IC
Time: September 2011 to February 2012
Project: “Qnint”
Advisor: Prof. Dr. Claudia Rezende
Institute of Chemistry
Jessica Silva dos Santos CV-Lattes
CNPQ Technical Support Grant– AT NM
From October to December 2010
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
Juliana Fátima Vilachã Madeira Rodrigues dos Santos CV Lattes
CNPq Technical Support Scholarship – IC
Time: March 2012 to Mar 2013
Project: “Planning, synthesis, and pharmacological evaluation of 1,2,3,4-tetrahydroacridine derivates, acetylcholonesterase inhibitor prototypes.”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Leandro Louback da Silva CV-Lattes
CAPES Doctoral Grant
October 2009 to August 2010
Project: “Study of the effects of different N-acylhydrazone derivatives on the cell-to-cell interaction mechanisms and inflammatory mediators that are part of the atherosclerotic process.”
Advisor: Prof. Dr. Ana Luisa Palhares de Miranda
LASSBio
Lidilhone Hamerski Carbonezi CV-Lattes
CNPq Junior Post-Doctorate Scholarship
Time: August 2010 to January 2011
Project: “Sunitinib synthesis”
Advisor: Prof. Dr. Angelo da Cunha Pinto
Institute of Chemistry (IQ)
Lucia Beatriz Torres CV-Lattes
CNPq Technological Development Scholarship – DTI-2
Time: October 2010 to September 2011
CNPq Technological Development Scholarship – DTI-1
Time: October 2011 to July 2012
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Luciana Almeida Piovesan CV-Lattes
CNPq Junior Post-Doctorate Scholarship
Time: February 2009 to August 2009
Project: “Design, Synthesis and Pharmacological Evaluation of Novel Anti-Cancer Drug-Candidate Prototypes”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Luciano da Silva Santos CV-Lattes
CNPq Scientific Initiation Scholarship - IC
Time: August to October 2011
CNPq Technical Support Scholarship – AT NS
Time: November 2011 to February 2012
Project: “Synthesis and pharmacological activity of new ferrocene-N-acylhydrazone derivates”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
Maria de Fátima do Nascimento Alfredo CV-Lattes
CNPq Technical Support Scholarship – AT NS
Time: January 2012 to June 2013
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Mariana Trad Rosner da Motta CV-Lattes
CNPq Scientific Initiation Scholarship - IC
Time: August to October 2011
Project: “In vitro metabolism of new leishmanicide and tripanomicide pharmaceutical prototypes”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
Marlon Daniel Tonin CV-Lattes
CNPq Technical Support Scholarship – DTI-3
Time: April 2012 to July 2012
Project: “Novel 5-aryl-2-furfuryl-N-acylhydrazone derivatives with potent anti-inflammatory and analgesic activity: LASSBio-1609 and LASSBio-1636”
Advisor: Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio
Natalia Medeiros de Lima CV-Lattes
CNPq Technical Support Scholarship – AT NS
Time: August 2010 to July 2011
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Pedro Gabriel Dias Lobato Pereira CV-Lattes
CNPq Scientific Initiation Scholarship - IC
Time: August to October 2011
Project: “Synthesis of cyclodextrin complexes of LASSBio-596 salts”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
Priscila de Paula Cabral CV-Lattes
CNPq Technological Development Scholarship – DTI-3
Time: May 2012 to June 2012
Project: “Scientific awareness and health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Raquel de Oliveira Lopes CV-Lattes
CNPq Technical Support Scholarship – DTI-3
Time: October 2010 to December 2010
Project: “Metabolic studies of LASSBio-596”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
Roberta Tesch CV-Lattes
CNPq Technical Support Scholarship – AT NS
Time: June 2010 to November 2010
CAPES Master Scholarship
Time: March to April 2011
Project: “Studies of molecular modeling and structural planning of new ligands to adenosine receptors”
Advisor: Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio
Rodolfo do Couto Maia CV-Lattes
CAPES Exchange Doctorate Scholarship (Dsw)
Time: February to July 2011
Project: “Synthesis and evaluation of antitumor activity of a new family of pyrazole-pyridone family"
Advisor: Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio
Tais Rubia dos Santos CV-Lattes
CNPq Scientific Initiation Scholarship - IC
Time: September to November 2011
Project: “Planning, synthesis and pharmacological evaluation of new leflunomide analogs”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
Thiago Stevanatto Sampaio CV-Lattes
CNPQ Technical Support Grant– AT NM
April 2009 to March 2010
Project: Design, synthesis and evaluation of cytotoxic properties of new TK inhibitor pharmaceutical candidate prototypes.
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
USP- RIBEIRÂO PRETO
Giuliana Bertozi Francisco CV-Lattes
CNPq Technical Support Scholarship – AT NM
Time: September 2010 to December 2011
Project: “Semicarbazone Benzaldehyde (BS)”
Advisor: Prof. Dr. Fernando de Queiroz Cunha
Faculty of Medicine of Ribeirão Preto
Ana Katia dos Santos CV-Lattes
CNPq Technical Support Scholarship – AT NM
Time: January 2012 to July 2013
Project: “Semicarbazone Benzaldehyde (BS)”
Advisor: Prof. Dr. Fernando de Queiroz Cunha
Faculty of Medicine of Ribeirão Preto
INCT-INOFAR Researchers
COORDINATOR
Eliezer J. Barreiro (UFRJ) CV-Lattes
VICE-COORDINATOR
Fernando de Queiroz Cunha (USP-RP) CV-Lattes
ASSOCIATED LABORATORY SUPERVISORS
Adelaide Maria de Souza Antunes (UFRJ) CV-Lattes
Angelo da Cunha Pinto (UFRJ) CV-Lattes
Carlos Alberto Manssour Fraga (UFRJ) CV-Lattes
Carlos Mauricio Rabello de Sant'Anna (UFRRJ) CV-Lattes
Claudio Viegas Junior (UNIFAL) CV-Lattes
Francisco Jose Roma Paumgartten (ENSP/FIOCRUZ) CV-Lattes
Francisco Radler de Aquino Neto (UFRJ) CV-Lattes
François Germain Noel (UFRJ) CV-Lattes
Gisele Zapata-Sudo (UFRJ) CV-Lattes
Heloisa de Oliveira Beraldo (UFMG) CV-Lattes
Joao Antonio Pegas Henriques (UFRGS) CV-Lattes
Jose Nelson dos Santos Silva Couceiro (UFRJ) CV-Lattes
Laurent Emmanuel Dardenne (LNCC) CV-Lattes
Lidia Moreira Lima (UFRJ) CV-Lattes
Luiz Carlos Dias (UNICAMP) CV-Lattes
Magna Suzana Alexandre Moreira (UFAL) CV-Lattes
Manoel Odorico de Moraes Filho (UFC) CV-Lattes
Marcia Paranho Veloso (UNIFAL) CV-Lattes
Marco Aurelio Martins (FIOCRUZ) CV-Lattes
Margareth de Fatima Formiga Melo Diniz (UFPB) CV-Lattes
Nelilma Correia Romeiro (UFRJ) CV-Lattes
Patricia Dias Fernandes (UFRJ) CV-Lattes
Patricia Machado Rodrigues e Silva Martins (FIOCRUZ) CV-Lattes
Patricia Rieken Macedo Rocco (UFRJ) CV-Lattes
Ricardo Menegatti (UFG) CV-Lattes
Roberto Takashi Sudo (UFRJ) CV-Lattes
Ronaldo de Albuquerque Ribeiro (UFC) CV-Lattes
Stela Maris Kuze Rates (UFRGS) CV-Lattes
Thereza Christina Barja Fidalgo (UERJ) CV-Lattes
Valeria de Oliveira (UFG) CV-Lattes
Vanderlan da Silva Bolzani (UNESP/ARARAQUARA) CV-Lattes
RESEARCHERS
Alberto Jose Cavalheiro (UNESP/Araraquara) CV-Lattes
Aloa Machado de Souza (UFRJ) CV-Lattes
Araken dos Santos Werneck Rodrigues (LNCC) – CV-Lattes
Antonio Carlos Doriguetto (UNIFAL) CV-Lattes
Bárbara Vasconcellos da Silva (UFRJ) CV-Lattes
Camila Silva de Magalhães (UFRRJ) CV-Lattes
Carolina Horta Andrade (UFG) CV-Lattes
Catarina de Nigris Del Cistia (UFRRJ) CV-Lattes
Carlos Alberto Tagliati (UFMG) CV-Lattes
Claudia Moraes de Rezende (UFRJ) CV-Lattes
Cristina Setim Freitas (USP/RP) CV-Lattes
Débora Gonçalves Xisto (UFRJ) CV-Lattes
Dulce Helena Siqueira Silva (UNESP/Araraquara) CV-Lattes
Edna Alves dos Anjos-Valotta (FIOCRUZ) CV-Lattes
Eduardo Cesar Zarzana (UFRGS) CV-Lattes
Fernanda Antunes (UENFE) CV-Lattes
Helio de Mattos Alves (UFRJ) CV-Lattes
Hélio José Correa Barbosa (LNCC) CV-Lattes
Henrique Marcelo Gualberto (UFRJ) CV-Lattes
Ian Castro-Gamboa (UNIFAL) CV-Lattes
Indianara Maria Araújo do Nascimento (UFRJ) CV-Lattes
Ingrid DraganTaricano (UFRGS) CV-Lattes
Izabel Vianna Villela (UFRGS) CV-Lattes
João Batista Neves da Costa (UFRRJ) CV-Lattes
José Ricardo Sabino (UFG) CV-Lattes
Katia de Angelis (UFRGS) CV-Lattes
Kênnia Rocha Rezende (UFG) CV-Lattes
Leoni Villano Bonamin (UFRGS) CV-Lattes
Leticia Veras Costa-Lotufo (UFC) CV-Lattes
Luciano Morais Lião (UFG) CV-Lattes
Magda Fraguas Serra (FIOCRUZ) CV-Lattes
Marcelo Henrique dos Santos (UNIFAL) CV-Lattes
Marcio de Matos Coelho (UFMG) CV-Lattes
Margarete Manhaes Trachez (UFRJ) CV-Lattes
Maria Regina Gomes Carneiro (FIOCRUZ) CV-Lattes
Mariana Lima Vale (UFC) CV-Lattes
Marize Campos Valadares Bozinis (UFG) CV-Lattes
Matheus Lavorenti Rocha (UFG) CV-Lattes
Miriana da Silva Machado (UFRGS) CV-Lattes
Newton Gonçalves de Castro (UFRJ) CV-Lattes
Pedro Leme Silva (UFRJ) CV-Lattes
Priscila Vanessa Zabala Capriles Golliat (LNCC) CV-Lattes
Priscilla Christina Olsen (FIOCRUZ) CV-Lattes
Raquel Carvalho Montenegro (UFC) CV-Lattes
Regina Maria Barretto Cicarelli (UNESP) CV-Lattes
Renato Sérgio Balão Cordeiro (FIOCRUZ) – CV-Lattes
Rodolfo do Couto Maia (UFRJ) CV-Lattes
Rosângela de Oliveira Alves Carvalho CV-Lattes
Sharlene Lopes Pereira (UFRJ) CV-Lattes
Tatiana Paula Teixeira Ferreira (FIOCRUZ) CV-Lattes
Thaiana da Cunha Ferreira Mendes (UFRJ) CV-Lattes
Thiago Mattar Cunha (USP/RP) CV-Lattes
Ulisses Gazos Lopes (UFRJ) CV-Lattes
Vinicius de Frias Carvalho (FIOCRUZ) CV-Lattes
Virginia Veronica de Lima (UFRJ) CV-Lattes
SCHOLARSHIPS
Adriano Siqueira Vieira (Unicamp) CV-Lattes
Allan Kardec Nogueira de Alencar (UFRJ) CV-Lattes
Alan Rodrigues de Sousa (UFRJ) CV-Lattes
Alexandra Basilio Lopes (UFRJ) CV-Lattes
Ana Carla Dos Santos (UFRJ) CV-Lattes
Ana Cristina da Mata Silva (UFRJ) CV-Lattes
Ana Katia dos Santos (UFRJ) CV-Lattes
Ana Maria Calçado dos Santos (UFG) CV-Lattes
André Victor Pereira (UNIFAL) CV-Lattes
Arthur Eugen Kümmerle (UFRJ) CV-Lattes
Arthur Henrique F. do Prado (UFRJ) CV-Lattes
Bárbara Assis Novak (UFRJ) CV-Lattes
Bruno Coelho Cavalcanti (UFC) CV-Lattes
Carlos Eduardo da Silva Monteiro (UFRJ) CV-Lattes
Carolina Neris Cardoso (UFMG) CV-Lattes
Clemilson Berto Junior (UFRJ) CV-Lattes
Daniel Nascimento do Amaral (UFRJ) CV-Lattes
Fabrício Maia da Silva Salvador (UFRJ) CV-Lattes
Gabrielle Luck de Araujo (UFMG) CV Lattes
Givanildo Santos da Silva (UFRJ) CV-Lattes
Giuliana Bertozi Francisco (USP-RP) CV-Lattes
Hannah Carolina T. Domingos (UFRJ) CV-Lattes
Jessica Silva dos Santos (UFRJ) CV-Lattes
Juliana Fátima Vilachã Madeira Rodrigues dos Santos (UFRJ) CV Lattes
Julio Beltrame Daleprane (FIOCRUZ) CV-Lattes
Leandro Louback da Silva (UFRJ) CV-Lattes
Leila de Souza Conegero (Unicamp) CV-Lattes
Lidilhone Hamerski Carbonezi (UFRJ) CV-Lattes
Lucia Beatriz Torres (UFRJ) CV-Lattes
Luciana Almeida Piovesan (UFRJ) CV-Lattes
Luciano da Silva Santos (UFRJ) CV-Lattes
Maria de Fátima do Nascimento Alfredo (UFRJ) CV-Lattes
Mariana Trad R. da Motta (UFRJ) CV-Lattes
Marlon Daniel Lima Tonin (UFRJ) CV-Lattes
Marcus Vinicius Dos Santos (UFRJ) CV-Lattes
Natalia Medeiros de Lima (UFRJ) CV-Lattes
Nathalia Freitas Emiliano (UFMG) CV-Lattes
Pedro Gabriel D. L. Pereira (UFRJ) CV-Lattes
Priscila de Paula Cabral (UFRJ) CV-Lattes
Raquel de Oliveira Lopes (UFRJ) CV-Lattes
Roberta Tesch (UFRJ) CV-Lattes
Rodolfo do Couto Maia (UFRJ) CV-Lattes
Samira de Sa e Souza (UFMG) CV-Lattes
Sarah da Silva Nunes (UFG) CV-Lattes
Tais Rubia dos Santos (UFRJ) CV-Lattes
Thiago Stevanatto Sampaio (UFRJ) CV-Lattes
Vinicius Frias de Carvalho (FIOCRUZ) CV-Lattes
Wallace Carvalho Ferreira (UFMG) CV-Lattes
INCT of Drugs and Medicines (INCT-INOFAR)
Coordinator
Eliezer J. Barreiro (LASSBio/UFRJ) CV-Lattes
Vice-coordinator
Fernando Queiroz Cunha (USP-Ribeirao Preto) CV-Lattes
Managing Committee
Angelo da Cunha Pinto (UFRJ) CV-Lattes
Heloisa de Oliveira Beraldo (UFMG) CV-Lattes
Luiz Carlos Dias (UINICAMP) CV-Lattes
Marco Aurelio Martins (FIOCRUZ-RJ) CV-Lattes
Vanderlan da Silva Bolzani (UNESP - Araraquara) CV-Lattes
Scientific Superintendence
Lidia Moreira Lima (LASSBio/UFRJ) CV-Lattes
Media Affairs
Fabricio Maia da Silva Salvador CV-Lattes
With the assistance of Lucia Beatriz Torres CV-Lattes
Executive Secretary
Ana Carla dos Santos CV-Lattes
Outreach Secretary
Ana Cristina da Mata Silva CV- Lattes
Finance Secretary
Edson de Almeida Naccor CV-Lattes
INCT-INOFAR Headquarters
UFRJ / Centro de Ciencias da Saude (CCS) – bloco K, sala 12
Cidade Universitaria – Rio de Janeiro/RJ
Cep: 21944971 Caixa Postal: 68073
Phone/Fax: +55 21 2562-6478