PRESENTATION
NATIONAL INSTITUTES OF SCIENCE AND
TECHNOLOGY PROGRAM (INCT)
In 2008, the Brazilian
government published the announcement MCT/CNPq
no014/2008 with a goal of recruiting scientists to work in
networks, in research areas strategic for the sustainable
development of the country. The public notice has been, so far, the
one to most greatly support Science and Technology in
Brazil.
At the time, part of the
scientists associated with the Millennium Institute of Innovation
and Development of Drugs and Medicines (IM-INOFAR) took on the
challenge and submitted a new project to the public notice of the
National Institutes of Science and Technology (INCTs). That is how
the Drugs and Medicines INCT was established (INCT-INOFAR).
As in the case of
INCT-INOFAR, 126 National
Institutes of Science and Technology (INCTs) have been established.
Articulating laboratories or associated research groups from
different parts in the country, INCTs have the mission of acting in
different areas of strategic importance for national
sovereignty. INCT-INOFAR
is in charge of health research aimed at the
discovery of new drugs and medicines.
DRUGS AND MEDICINES INCT (INCT-INOFAR)
The National Institute of
Science and Technology of Drugs and Medicines (INCT-INOFAR) is a research network
that brings together renowned scientists from different research
institutions and universities in Brazil. Its mission is to act in
the discovery of new drugs and medicines, as well as new synthesis
routes for generic drugs, and also to work for the professional
education of graduate and undergraduate students in Medicinal
Chemistry and Pharmacology, key disciplines for the process of drug
discovery.
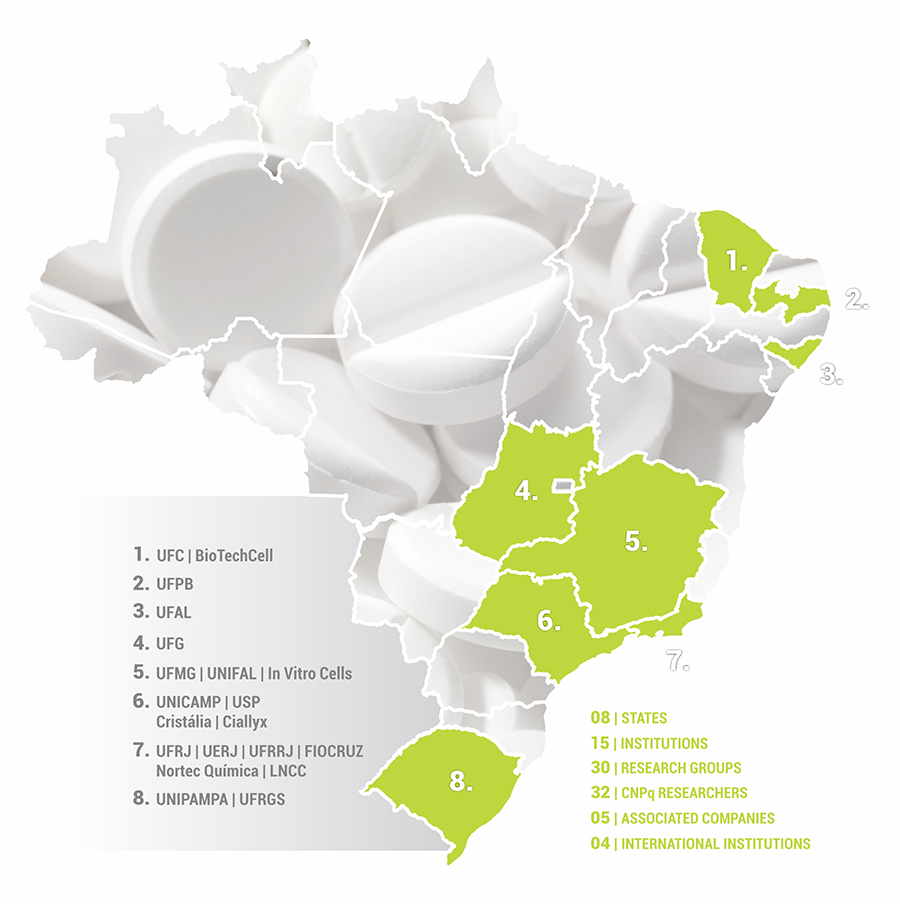
Made up of nearly one
hundred scientists from 30 research groups with efforts focused on
radical pharmaceutical innovation and incremental innovation in
generic drugs, INCT-INOFAR is present in 15 teaching
and research institutions in 8 different Brazilian
states.
With a task of qualifying
personnel to work in important stages of the process of
discovery/invention of new drugs from the choice of therapeutic
target to the conclusion of pre-clinical bioassays -
INCT-INOFAR contributes to
the identification and solving important bottlenecks in the chain
of pharmaceutical innovation.
Parallel to the laboratory
research, INCT-INOFAR also acts in society, promoting science and encouraging the
rational and responsible use of drugs through health education
actions. It also maintains the Drugs
Portal, a website created to
promote Pharmaceutical Sciences in the academic community and in
society at large.
MISSION
- To organize national scientific
competencies in an effective and productive network of research in
drugs and medicines;
- To support scientific research subprojects
in the chain of innovation in drugs and medicines;
- To act in incremental innovation in drugs
through generics;
- To study and develop new total synthesis
routes for current and future generic drugs, advanced intermediates
and strategic raw materials for the sector;
- To contribute for the scientific
qualification of personnel in Medicinal Chemistry &
Pharmacology;
- To promote scientific awareness
related to the use of drugs and medicines, therefore contributing
effectively for their rational and safe use.
INVESTING IN RADICAL AND INCREMENTAL
PHARMACEUTICAL INNOVATION
With the contribution of
its entire research network, INCT-INOFAR studies and develops
several radical innovation subprojects and also acts in incremental
innovation, studying new total synthesis routes for generic
drugs.
In the field of radical
innovation, the Institute aims to discover/invent original
substances, active in in vivo
widely validated pharmacological models able to
originate new drug candidates in several pharmaceutical classes.
The different research areas of interest to INCT-INOFAR are: inflammation,
pulmonary diseases, pain, central nervous system, cardiovascular
system and chemotherapy of cancer and of neglected diseases,
particularly leishmaniasis.
In the field of incremental
innovation, INCT-INOFAR leads projects that are focused on the search for new
synthetic routes, efficient and accessible, for generic drugs
already in the market as well as for those about to have their
patent protections expired, representing, by their market share,
new business opportunities for the Brazilian pharmaceutical
sector.
INNOVATION IN NEW GENERIC
ROUTES
In spite of advances after
nearly 14 years of the Law of Generic Drugs
(no9.787/1999) in Brazil, unfortunately so far Brazilian
pharmaceutical companies as a whole are limited to formulating and
packaging active principles important from far away markets like
China, India, and Korea. Working hard to try to reverse this
Indian Pathway process, INCT-INOFAR
makes efforts in the
study and development of total synthesis routes for generic drugs
with a goal of transferring the technology acquired to the local
industry.
By studying and developing
total synthesis routes of generic drugs, advanced intermediates and
strategic raw materials for the sector, INCT-INOFAR researchers pave the way
for the production of active principles of drugs that are important
instruments for health care policies and for the population. Since
its creation, in 2009, INCT-INOFAR has already developed new
synthesis routes for the active principles of three
drugs.
ATORVASTATIN
In the same month when the
patent for Lipitor™/Pfizer expired in Brazil (December, 2010),
INCT-INOFAR researchers
announced the discovery of a new synthesis route for its active
principle, atorvastatin. A continuous use drug for cholesterol
reduction, Lipitor™has been the best-selling drug
in history. The synthesis route of atorvastatin has been patented,
and represents an important technological asset for
INCT-INOFAR, which has
been trying to negotiate the production of this generic drug with a
Brazilian pharmaceutical company ever since.
SUNITINIB
Recommended for certain
types of stomach cancer, sunitinib is the active principle of
Sutent™/Pfizer, a high cost drug, which is unfortunately not yet
available in the Public Health Care System (SUS) and that is,
therefore, the subject of several court cases, as it is the primary
drug recommended for these cases. The sunitinib synthesis route was
completed by INCT-INOFAR in September 2011. With the discovery,
Brazil can prepare to produce the medication before the patent for
the drug expires, reducing production cost.
FLUOXETINE
Antidepressant drug from
the selective serotonin reuptake inhibitor class, fluoxetine was
marketed by Eli Lilly under the name Prozac™, until its patent expired in
Brazil, in 2001. Considered the controlled drug with the highest
demand in the Public Health Care System, most fluoxetine consumed
in Brazil is imported. Considering the social and market impacts of
this drug, the technological know-how of the fluoxetine synthesis
is an important INCT-INOFAR achievement.
MULTIDISCIPLINARY RESEARCH
NETWORK
The process of innovation in drugs has clear
interdisciplinary and multidisciplinary characteristics, demanding
competencies in distinctive areas of Health Sciences.
INCT-INOFAR brings together, in a network, research groups of
academic-scientific excellence, in different areas, covering all
stages of the process of invention of new drugs, ranging from the
election of the therapeutic target to the conclusion of
pre-clinical stage bioassays, quantitative and qualitative
analytical methods, as well as clinical pharmacology.
The INCT-INOFAR multidisciplinary team is
made up of experts in different subjects, like Medicinal Chemistry,
Pharmacology, Organic Chemistry, Toxicology, Organic Synthesis,
Biochemistry, Computational Chemistry, Structural Biology,
Spectroscopy, and Chemistry of Natural Products, among other
related areas.
SCIENTIFIC
EXCHANGE
Present in 15 teaching and
research institutions, in eight different Brazilian states,
INCT-INOFAR has actively
contributed to diminish the regional scientific imbalance in
Brazil, as well as to increase national expertise in a sector
strategic to the country.
By making it possible for
researchers from different institutions, in different geographical
areas, to work together, INCT-INOFAR establishes an exchange
between large centers and emerging research groups.
Cooperation is a way
for INCT-INOFAR to
contribute toward the increasing of scientific and technological
production in emerging centers, especially in the Northeast and
Midwest regions, benefitting the formation of undergraduate and
graduate students in the field. Throughout the past 5 years, the
scientific advancement of these emerging groups was
notable.
INCT-INOFAR RESEARCH
GROUPS: LABORATORIES AND PERSONNEL IN CHARGE
Network
Coordinator: Prof. Eliezer J.
Barreiro (LASSBio/UFRJ) Cv Lattes
Rio de
Janeiro
-
FIOCRUZ
Laboratory of
Inflammation (IOC)
Marco Aurelio
Martins CV-Lattes
Laboratory of Environmental Toxicology (ENSP)
Francisco Jose
Roma Paumgartten CV-Lattes
-
UERJ
Department of Pharmacology(IBRAG)
Theresa Christina
Barja-Fidalgo CV-Lattes
-
UFRJ
Laboratory of Evaluation and Synthesis of Bioactive
Substances LASSBio (ICB)
Carlos Alberto Manssour Fraga CV-Lattes
Lidia Moreira Lima
CV-Lattes
System of Information on the Chemical Industry SIQUIM (EQ)
Adelaide Maria de Souza Antunes CV-Lattes
Laboratory of Pulmonary Investigation (IBCCF)
Patricia Rieken Macedo Rocco CV-Lattes
Laboratory of Biochemical and Molecular Pharmacology
(ICB)
Francois
Germain Noel CV-Lattes
Laboratory of Cardiovascular Pharmacology (ICB)
Gisele
Zapata Sudo CV-Lattes
Laboratory of Muscular Excitation-Contraction
Coupling (ICB)
Roberto Takashi
Sudo CV-Lattes
Laboratory of Natural Products and Chemical
Transformations (IQ)
Angelo da Cunha Pinto CV-Lattes
Laboratory of Support to Technological
Development (IQ)
Francisco Radler de Aquino Neto CV-Lattes
Laboratory of Pharmacology of Pain and
Inflammation (ICB)
Patricia Dias
Fernandes CV-Lattes
-
UFRRJ
Institute of Exact Sciences (IQ)
Carlos Mauricio Rabello de Santa´Anna
CV-Lattes
-
LNCC-MCTI
Group of Molecular Modelling of Biological
Systems (Department of Computational Mechanics)
Laurent
Emmanuel Dardenne CV-Lattes
Sao
Paulo
-
USP
Laboratory of Pain and Inflammation (Faculty
of Medicine - Ribeirao Preto)
Fernando de Queiroz Cunha CV-Lattes
Laboratory of Design and
Synthesis of Chemotherapeuticals Potentially Active on Neglected
Diseases (Faculty of Pharmaceutical Sciences - Sao
Paulo)
Elizabeth
Igne Ferreira
CV-Lattes
-
UNICAMP
Laboratory of Synthetic Organic Chemistry
(IQ)
Luiz Carlos
Dias CV-Lattes
Minas
Gerais
-
UFMG
Group of Innovation in Organic and Inorganic
Compounds with Pharmacological Activity (Department of
Chemistry)
Heloisa de Oliveira Beraldo CV-Lattes
Laboratory of Experimental
Toxicology (in vitro andin vivo)
Carlos Alberto Tagliati CV-Lattes
-
UNIFAL
Laboratory of Phytochemistry and Medicinal
Chemistry (Faculty of Pharmacy)
Claudio
Viegas Junior CV-Lattes
Agency of Innovation and Entrepreneurship
(Dean of Graduate School and Research)
Marcia Paranho
Veloso CV-Lattes
Rio Grande de
Sul
-
UFRGS
Laboratory of Experimental
Psychopharmacology (Faculty of Pharmacy)
Stela Maris Kuze Rates
CV-Lattes
-
UNIPAMPA
Laboratory of Pharmacology LABFAR (Faculty of
Pharmacy)
Sandra Elisa
Haas CV-Lattes
Goias
-
UFG
Laboratory of Bioconversion (Faculty of
Pharmacy)
Valeria de Oliveira
CV-Lattes
Laboratory of Medicinal Pharmaceutical
Chemistry (Faculty of Pharmacy)
Ricardo
Menegatti CV-Lattes
Alagoas
-
UFAL
Laboratory of Pharmacology and Immunity (Institute of
Biological and Health Sciences)
Magna Suzana
Alexandre Moreira CV-Lattes
Ceara
-
UFC
Unit of Clinical Pharmacology (Faculty of
Medicine)
Manoel Odorico
de Moraes CV-Lattes
Laboratory of Pharmacology of Inflammation
and Cancer (Faculty of Medicine)
Ronaldo de
Albuquerque Ribeiro CV-Lattes
Department of Physiology and Pharmacology
(Faculty of Medicine)
Claudia do Ó
Pessoa CV-Lattes
Paraiba
-
UFPB
Laboratory of Toxicological Assays LABETOX
(Department of Pharmaceutical Sciences)
Margareth de Fatima Formiga Melo Diniz
CV-Lattes
MAP OF RESEARCH NETWORK
QUALIFICATION OF PERSONNEL
Collaborating to the
enhancement of Brazilian expertise in the discovery/invention of
new drugs and medicines, INCT-INOFAR
works strongly in the qualification of personnel
in the several research centers associated with it.
At INCT-INOFAR, scientific qualification is improved at different academic
levels: undergraduate, Master's Degree, Doctorate, and
Post-Doctorate. As part of this qualification, graduate students
connected to the projects under study are encouraged to take part
in scientific exchange with participating laboratories with
specific expertise, so as to meet the agreed goals in adequate
time.
Through scientific exchange
promoted and encouraged by INCT-INOFAR, the Institute contributes
not only for the qualification of new researchers, but also to the
continuing education and updating of senior researchers. Keeping
talented professionals in the country is also an
INCT-INOFAR goal.
- Training of personnel
- Academic-scientific exchange
- Continuing education and updating of
senior researchers
- Keeping talented researchers in the
country
COOPERATING TO IMPROVE
GRADUATE EDUCATION IN THE COUNTRY
INCT-INOFAR
researchers actively take part in personnel
qualification activities, through membership in 35 prestigious
Graduate Programs, throughout the country, most of them offered at
both the Master's and Doctorate levels. Over half of the Graduate
Programs with the participation of INCT-INOFAR researchers are classified
at excellence grades 6 and 7 (out of a maximum 7) by the Commission
for the Improvement of Higher Education
Personnel (CAPES).
CAPES is an agency of the
Ministry of Education responsible for ranking and evaluation
Stricto Sensu Graduate
Programs (Academic Master's Degree, Professional Master's Degree,
and Doctorate) in the country.

The process of evaluation
of Graduate Programs conducted by CAPES is continuous. The course
must be evaluated every three years (triennial evaluations) to
assess if the goals proposed in the initial project were fully
achieved within the Program, earning the corresponding grades
ranging from 2 to 7.
GRADUATE PROGRAMS
INVOLVING INCT-INOFAR RESEARCHERS
CAPES GRADE 7
- Graduate
Program in Cellular and Molecular Biology
(FIOCRUZ) - M/D Levels
- Graduate
Program in Pharmaceutical Sciences (UFRGS) - M/D Levels
-
Graduate Program in Pharmacology (USP/RP) - M/D Levels
- Graduate
Program in Chemistry (UFMG) - M/D
Levels
- Graduate Program in
Chemistry (UFRJ) - M/D
Levels
- Graduate Program in Chemistry
(UNICAMP) - M/D Levels
CAPES GRADE 6
- Graduate Program in Pharmacology (UFC) - M/D Levels
-
Graduate Program in Computational Modelling
(LNCC) - M/D
Levels
-
Graduate Program in Public Health
(FIOCRUZ) - M/D Levels
CAPES GRADE 5
- Graduate Program
in Animal Sciences (UFG) -
M/D Levels
- Graduate
Program in Pharmaceutical Sciences (UFMG) - M/D Levels
- Graduate Program in Pharmacology and Medicinal Chemistry
(UFRJ) - M/D
Levels
- Graduate
Program in Neurosciences (UFRGS) - M/D Levels
-
Graduate Program - Northeast Network in Biotechnology
(RENORBIO) - D
Level
- Graduate Program in Biopharmaceutical Innovation
(UFMG) - F
Level
CAPES GRADE 4
- Graduate Program in Clinical and Toxicological Analyses
(UFMG) - M/D
Levels
- Graduate
Program in Computational Biology and Systems -
(FIOCRUZ) - M/D Levels
- Graduate Program
in Biotechnology - (UFC) -
M Level
- Graduate Program in Cardiology (UFRJ) - M/D Levels
- Graduate Program in Surgical Sciences
(UFRJ) - M/D
Levels
-
Graduate Program in Health Sciences
(UFAL) - M/D
Levels
- Graduate
Program in Pharmaceutical Sciences (UFG) - M Level
- Graduate Program in Pharmaceutical Sciences
(UNIFAL) - M/D
Levels
- Graduate Program in Pharmacology and Therapeutic -
(UFRGS) - M/D
Levels
-
Graduate Program in Drugs and Medicines
(USP) - M/D
Levels
- Graduate
Program in Physics (UFG) -
M/D Levels
- Graduate
Program in Pharmaceutical Innovation - (UFG) - M/D Level
- Graduate Program in
Mathematical and Computational Modelling (UFRRJ)
- M Level
- Graduate Program
in Nanosciences and Advanced Materials
(UFABC) - M/D
Levels
-
Graduate Program in Chemistry (IME) - M/D Levels
- Graduate
Program in Chemistry (UFRRJ) -
M/D Levels
- Graduate Program in Chemistry (UNIFAL) - M/D Levels
- Graduate Program in Intellectual Property and Innovation (
INPI ) - F
Level
CAPES GRADE 3
- Graduate Program in Pharmaceutical Sciences
(UNIPAMPA) - M
Level
-
Graduate Program in Pharmaceutical Sciences
(UFAL) - M
Level
*Source: Triennial Evaluation Report 2013 Reference
2010-2013, CAPES.
See full list of Master's
degree and doctoral theses advised by INCT-INOFAR researchers completed in
2014 on chapter 5 of this
publication.
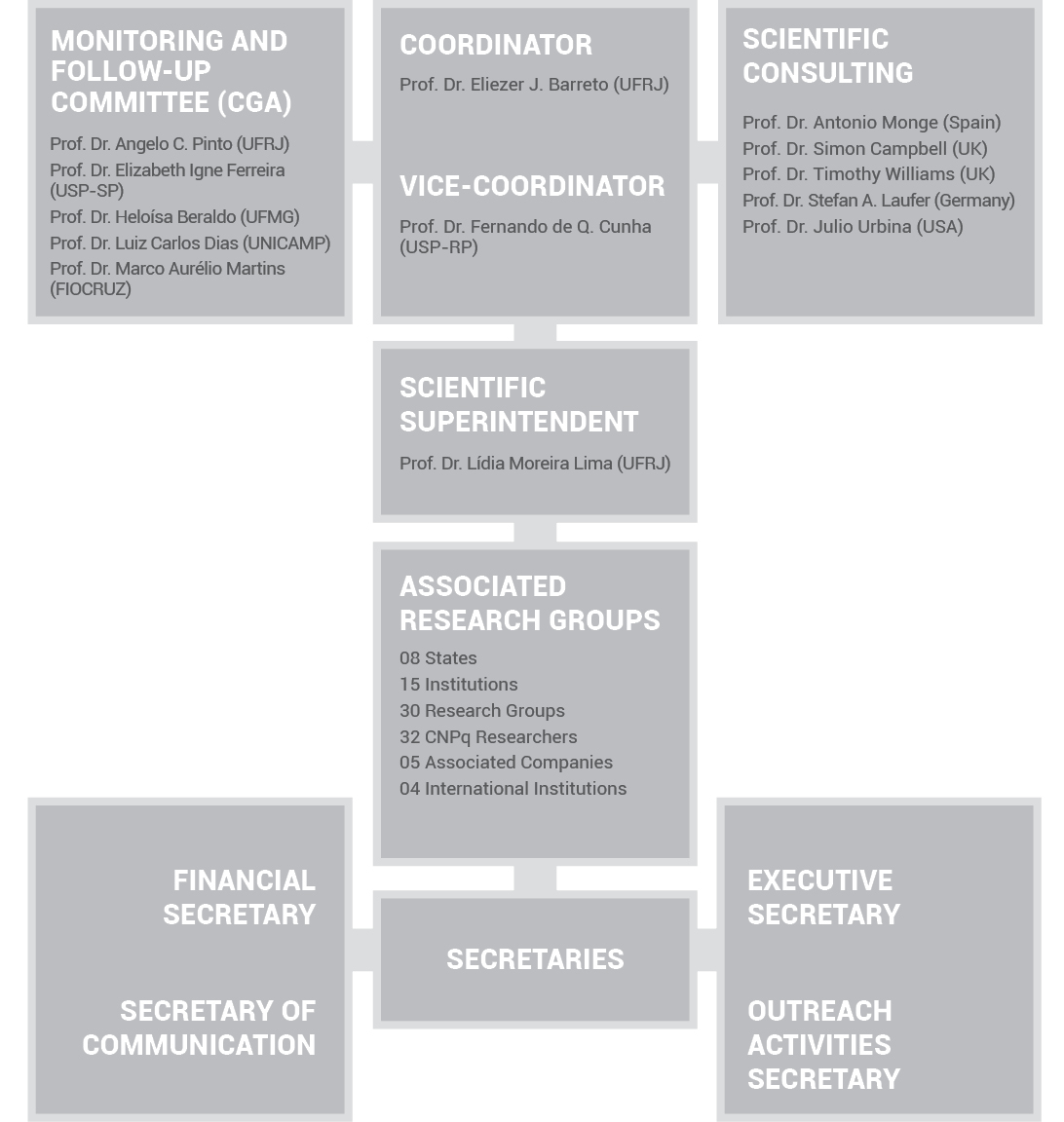
ORGANIZATIONAL STRUCTURE
The organizational structure
of INCT-INOFAR is
made up of a Coordinator, a Vice-Coordinator, and the Monitoring
and Follow-Up Committee (CGA). The CGA is a consulting and
deliberative collegiate, which acts in the strategic planning
of INCT-INOFAR activities.
The Scientific Superintendence supports the
Coordination, acting both in the technical scientific evaluation of
projects under study, and on the meeting of previously established
deadlines for the goals.
INCT-INOFAR
also has the participation, under confidentiality,
of expert consultants who provide scientific assistance in the
evaluation of projects under study, to optimize research
activities. In a few projects, consultants suggest possible route
changes needed to fulfill the ultimate goal of the Institute:
contributing toward the discovery of new Brazilian drugs.
The INCT-INOFAR scientific competences
network is made up of 30 different research groups located in 15
institutions in 8 different Brazilian states. Each
INCT-INOFAR associate
research group is led by an expert, responsible for the scientific
interaction of his or her team and with the other teams of the
Institute.
Secretaries of Finances,
Communication, and Outreach Activities, as well as Executive
Secretary support the full development of the research and
education activities of INCT-INOFAR, and are physically located
at the Center for Health Sciences (CCS) of UFRJ, the administrative
headquarters of the Institute.
INCT-INOFAR
ORGANIZATION CHART
With a goal of establishing
strong cooperation in the areas of management, research, and
scientific awareness, INCT-INOFAR periodically meets with
other National Institutes of Science and Technology
(INCTs).
The coordinators forum,
called I5 + (due to the fact that it was organized by five INCTs
that initially got together in late 2009 to start discussions),
creates documents who are sent to CNPq with concrete proposals to
solve operational, bureaucratic, and funding issues, referring to
the Institutes funded by the INCTs program.

ASSOCIATED COMPANIES
INCT-INOFAR has the support, even if
informal, of pharmaceutical and related companies, like In Vitro
Cells - Toxicological Research PLC, Cristalia Chemical and
Pharmaceutical Products Ltd., Ciallyx Laboratories & Consulting
Ltd., BiotechCell, and Nortec Chemistry.
IN VITRO CELLS TOXICOLOGICAL RESEARCH
PLC
In Vitro Cells - Toxicological Research PLC
is a technology company located at Biominas Foundation (Belo
Horizonte, MG). The founders are Professors at the Federal
University of Minas Gerais (UFMG) in the fields of Toxicology and
Biochemistry. The company is an INCT-INOFAR partner to conduct in
vitro bioassays to evaluate the safety and efficacy of new drug
candidates developed by the Institute.
CRISTALIA CHEMICAL PHARMACEUTICAL PRODUCTS
LTD.
Cristalia Chemical Pharmaceutical Products
Ltd. is a pharmaceutical company associated to INCT-INOFAR,
qualified to support possible future stages of pharmaceutics
development of new compound-prototypes that reach this advanced
stage in the chain of innovation in drugs and medicines. Under
non-disclosure and confidentiality agreements, Cristalia will
benefit, if there is interest, of information on the projects under
study, by expressing it during the appropriate timelines in
internalizing the technologies developed by INCT-INOFAR. For
technology transferring, the UFRJ Agency of Innovation and its
equivalent in another research institution connected to INCT-INOFAR and to a specific project will negotiate directly with the
interested parties, including the funders.
CIALLYX LABORATORIES & CONSULTING
LTD.
Ciallyx Laboratories & Consulting Ltd.
is a company housed at CIETEC (Center for Incubation of
Technological Companies), which carries out efficacy studies
(proofs of concept) and safety studies (toxicological studies and
assays) for new molecules, drugs, and formulations. Ciallyx
generates results according to national and international protocols
under strict quality parameters, using, as a guide, the
international norms of Good Laboratory Practices GLP. The company
is an INCT-INOFAR partner to conduct in vivo bioassays of safety
and efficacy of new drug candidates developed by the Institute.
BIOTECHCELL
The term Biotechnology refers to a wide set
of enabling and potentializing technologies that involve the use,
controlled alteration and optimization of living organisms or their
derivates, like cells and molecules, for the generation of
processes and services. BiotechCellR is a biotechnology
entrepreneurial company in the Northeast, born out of the
scientific community from the ideal of a pair of young researchers
who intended to align their vast academic experience to the
management of technological innovation and services. It is an
INCT-INOFAR partner that acts in research and pre-clinical
pharmacological services, human biomonitoring, toxicogenetics, and
applied toxicology.
NORTEC CHEMISTRY
In the process of pharmaceutical innovation,
the active principle is fundamental for the construction of new
synthesis routes. Nortec Chemistry is a 100% Brazilian
pharmachemical company, with a stated intent of acting in
partnership with INCT-INOFAR in the production of pharmaceutical
active principles. Nortec Chemistry, established in the 1980s, is
headquartered in Rio de Janeiro (RJ) and has, for several years in
a row, received the Prize of Excellence in Supplying Raw Materials,
awarded by SINDUSFARMA Union of the Pharmaceutical Industries of
Sao Paulo.
INTERNATIONAL AGREEMENTS
INCT-INOFAR
has directed efforts toward making its research
network international, through signing cooperation agreements with
international institutions. This is in accordance to the
recommendations of the National Council for Scientific and
Technological Development (CNPq) and the philosophy of the Science
Without Borders program, supported by this financial
agency.
The goal is to establish
international visibility to Science, Technology, and Innovation
activities in Brazil, and, most of all, allowing new cooperation
networks is built, and that those may offer training opportunities
for undergraduate and graduate students abroad. Currently,
INCT-INOFAR has cooperation
agreements with four Teaching and Research Institutes abroad,
allowing for the exchange of its researchers with experts in
Germany, Portugal, Italy, and Uruguay.

INCT-INOFAR INTERNATIONAL
COOPERATION NETWORK

GERMANY
Interdisciplinary Center of Pharmacogenomics
and Pharmaceutical Research (ICEPHA)
University of
Tübingen, Germany.
Researcher in Charge: Professor Stefan
Laufer

PORTUGAL
Department of Chemistry
University of
Aveiro, Portugal.
Researcher in Charge: Professor
Jose A. F. Cavalheiro

ITALY
Department of Pharmaceutical Sciences
University of
Ferrara, Italy.
Researcher in Charge: Professor Pier G.
Baraldi

URUGUAY
Department of Organic Chemistry
National University of the
Republic, Uruguay.
Researchers in Charge: Professors Hugo
Cerecetto and Mercedes Gonzalez
Among the main goals of international
agreements are the development of joint research projects,
organization of academic and scientific activities, exchange of
researchers and/or students, as well as exchange of materials and
publications relevant to the area.
ANNUAL "GERMANY + BRAZIL" SYMPOSIUM FOR INDIVIDUALIZED MEDICINE
In the year when Brazil and
Germany straightened their links through the "Brazil + Germany"
2013-2014 Year, comprising several events in Brazil,
INCT-INOFAR was part of the
workshop on Individualized Medicine in drug research.

Individualized or personalized therapy
oppose the classic drug development and therefore are not dominant
in the pharmaceutical industry. However, they have been strongly
investigated by academic workgroups, among them the
Interdepartmental Center for Pharmacogenomics and Drug Research
(ICEPHA), of the University of Tübingen.
The event, which took place
in the Brazilian Academy of Sciences (ABC), on March
28th,
2014, had the participation of Brazilian and German researchers,
with the goal of effectively developing projects in a
network.
The Workshop organized by
Professor Dr. Stefan Laufer, of ICEPHA/University of Tübingen, is one of the fruits of the
cooperation covenant that was signed in 2011 between the German
research institute and INCT-INOFAR, with the approval of
Prime Minister Winfried_Kretschmann and of Minister Theresia Bauer (Minister of
Science, Research, and Art, Baden-Württemberg).
OTHER INTERNATIONAL
ACTIONS
Parallel to international agreements, INCT-INOFAR makes efforts to establish eventual collaborations between
its researchers and renowned foreign scientists. Under
confidentiality, INCT-INOFAR has the
participation of international consultants who provide scientific
assistance in the evaluation of projects under study. Currently,
the Institute has three international
consultants:
INCT-INOFAR International
Scientific Consultants
- Sir Simon Campbell
(Pfizer, Royal Academy of
Science/England)
- Professor Antonio Monge
(University of Navarra, Spain)
- Dr. Camille G. Wermuth
(Prestwick Chemical, France)
INCT-INOFAR SUBPROJECTS UNDER STUDY
RADICAL
INNOVATION
INFLAMMATION
(Pulmonary Disease)
- Study of the potential anti-inflammatory effect of
LASSBio 897 compound, in silicosis and asthma models
Professor
Patricia Machado Rodrigues e Silva Martins (FIOCRUZ RJ)
CV-Lattes
Professor
Marco Aurelio Martins (FIOCRUZ RJ) CV-Lattes
- Study for the identification of new sulfonamide
compounds effective in the control of pulmonary inflammation caused
by silica in mice
Professor
Patricia Machado Rodrigues e Silva Martins (FIOCRUZ-RJ)
CV-Lattes
- Development of new antiasthmatic drug prototypes
(LASSBio-596)
Professor Patricia
Rieken Macedo Rocco (UFRJ) CV-Lattes
Professor
Lidia Moreira Lima (UFRJ) CV-Lattes
- Impact of therapy with nanoparticles of the
thymuline gene in chronic allergic asthma model
Professor Patricia Rieken
Macedo Rocco (UFRJ) CV-Lattes
INFLAMMATION AND PAIN
- New
5-aryl-2-furfuril-N-acylhydrazone functionalized derivatives with powerful
anti-inflammatory and analgesic action: LASSBio-1609 and
LASSBio-1636
Professor Carlos
Alberto Manssour Fraga (UFRJ) CV-Lattes
- Development of new anti-arthritis drug candidates,
MAPK p-38 modulators
Professor
Lidia Moreira Lima (UFRJ) CV-Lattes
- Design, synthesis, structural characterization and
pharmacological evaluation of new anti-inflammatory,
anti-infection, and neuroactive drug candidates
Professor Claudio Viegas
Junior (UNIFAL) CV-Lattes
- Development of new anti-inflammatory and analgesic
drug candidates from safrole
Professor
Lidia Moreira Lima (UFRJ) CV-Lattes
- Design of structural changes
aimed at optimizing the affinity of the selective IKK2 enzyme
inhibitor LASSBio-1524
Professor
Laurent Emmanuel Dardenne
(LNCC) CV-Lattes
- Benzaldehyde
semicarbazone (BS)
Professor
Heloisa de Oliveira Beraldo(UFMG) CV-Lattes
CHEMOTHERAPY
- Evaluation of antiparasitic
activity of a series of semicarbazone and
hydrazine-N-acylhydrazone derivatives (Leishmanicidal)
Professor Magna Suzana
Alexandre Moreira (UFAL) CV-Lattes
- Discovery of new antitumoral drug
candidate analogs to combrestatin A4 (Antineoplastic)
Professor
Lidia Moreira Lima (UFRJ) CV-Lattes
- Theoretical investigation of
action of dialkylphosphorilhydrazones as ribose 5-phosphate
isomerase enzyme of Trypanosoma
cruzi and Plasmodium falciparum (Trypanomicidal and
antimalarial)
Professor Carlos
Mauricio R. de Sant' Anna (UFRRJ) CV-Lattes
CENTRAL NERVOUS SYSTEM
- Study of N-phenylpiperazine functionalized
derivatives as prototypes for the development of new atypical
antipsychotics (antipsychotics)
Professor Stela Maris Kuze
Rates (UFRGS) CV-Lattes
Professor Carlos
Alberto Manssour Fraga (UFRJ) CV-Lattes
- Pharmacological evaluation of new
Zolpidem neuroactive derivatives (neuropathic pain)
Professor Roberto
Takashi Sudo (UFRJ) CV-Lattes
- Design, synthesis and pharmacological evaluation of
vectorized and self-organized neuroactive drug prototypes
Professor Ricardo
Menegatti (UFG) CV-Lattes
CARDIOVASCULAR SYSTEM
- Therapeutic potential of new vasodilator (LASSBio
1289) in arterial and pulmonary hypertension
Professor Gisele Zapata
Sudo (UFRJ) CV-Lattes
- Pharmacological and toxicological evaluation of new
drug candidates for the prevention and treatment of miocardiopathy
and neuropathy caused by diabetes mellitus
Professor Gisele Zapata
Sudo (UFRJ) CV-Lattes
INCREMENTAL
INNOVATION
GENERICS
- Synthesis of Quetiapine
Professor
Eliezer J. Barreiro (UFRJ) CV Lattes
Professor
Angelo da Cunha Pinto (UFRJ) CV Lattes
- Synthesis of Fluoxetine
Professor
Eliezer J. Barreiro (UFRJ) CV Lattes
Professor
Luiz Carlos Dias (UNICAMP) CV Lattes
Dr. Adriano
V. Siqueira (UNICAMP) CV Lattes
- Synthesis of Valsartan
Professor
Eliezer J. Barreiro (UFRJ) CV Lattes
Professor
Luiz Carlos Dias (UNICAMP) CV Lattes
2014 HIGHLIGHTS
INCT-INOFAR HELPS INCREASE FEMALE RECOGNITION IN
SCIENCE
INCT-INOFAR RESEARCHER TAKES SEAT ON BRAZILIAN ACADEMY OF SCIENCES
Adding to the female presence in the Brazilian Academy of Sciences
(ABC), Professor Heloisa de Oliveira Beraldo, of the Federal
University of Minas Gerais (UFMG), took seat on May 06 as a full
member in the field of Chemistry. Professor Heloisa, who is
an INCT-INOFAR associate researcher and
also a member of its Managing Committee, was one of four women to
join the ranks of the nearly century-old Academy in
2014.
Professor Dr. Heloisa de Oliveira Beraldo
has made important contributions to Inorganic Medicinal Chemistry,
with studies of drug candidates and metallodrug candidates, among
them antitumor, antimicrobial, and antiparasitic. The Professor was
also responsible for the evaluation of pharmacological profiles of
different compounds and investigated the action and interaction
mechanism between organic compounds and metallic complexes with
target biomolecules, such as DNA and enzymes/metalloenzymes.

Professor Heloisa Beraldo receives title of
full member of the Brazilian Academy of Sciences from the Minister of Science, Technology, and
Innovation
Working hard to break the
historical paradigm of the male hegemony at the Brazilian Academy
of Sciences (ABC), INCT-INOFARis responsible for
increasing, yearly, the number of female scientists as full
members, especially in the field of Chemical Sciences. In 2012,
INCT-INOFAR associate researcher Professor
Vanderlan da Silva Bolzani, from State University of Sao Paulo
(UNESP-Araraquara), also became a member of the Academy.
YOUNG INCT-INOFAR RESEARCHER RECEIVES "FOR WOMEN IN SCIENCE"
AWARD
Young INCT-INOFAR researcher Professor
Dr. Carolina Horta Andrade, from the Federal University of Goias
(UFG), was the 2014 winner of the "For Women in Science" Award in
Chemistry. The research was on new multitarget drug candidates and
metallodrug candidates for Leishmaniasis, and was awarded a prize
of 20 thousand dollars.

Youngest of a family of five
women, Carolina was born in Formosa, in the state of Goias, and
dedicated herself to studying from an early age. The passion for
Medicinal Chemistry happened soon after graduating from the Federal
University of Goias (UFG), where she was advised by Professor Dr.
Valeria de Oliveira (UFG), an INCT-INOFAR associate researcher.
After graduating, she decided to get her Master's Degree at the
University of Sao Paulo (USP) with Professor Dr.
Elizabeth Igne Ferreira, member of the
INCT-INOFAR Managing
Committee. An expert in the field, Professor Elizabeth Ferreira
noticed Carolina Horta's excellent performance, and nominated her
for a doctorate consecutively, before she had even finished her
Master's Degree.

Carolina Andrade was the first researcher
from Goias to receive the "For Women in Science" Award
Currently at 31 years old, Carolina has a
vast academic background, including an exchange doctoral program at
the University of New Mexico, in Albuquerque, USA. To her, the
award is an incentive to young women to choose careers in science.
The award encourages not only local scientific production, but
also young female doctors who are beginning their careers in
Brazilian research institutions, she stated.
UNICAMP RESEARCHER HAS MERIT IN CHEMISTRY
ACKNOWLEDGED
Coordinator of the
INCT-INOFAR scientist group
who developed a cheaper process for the production of atorvastatin,
the active principle of Lipitor™, continuous use drug for the
control of cholesterol best sold in the world, Professor Dr. Luiz
Carlos Dias, from the Department of Organic Chemistry from the
State University of Campinas (UNICAMP), was the winner of the 2014
edition of the Walter Borzani Award.
Promoted by the Regional Council of
Chemistry (CRQ-IV), the contest intends to acknowledge
professionals who made significant contributions to their areas and
to the development of Chemistry.
The ceremony for the Walter Borzani Award
took place on June 7, at the Council headquarters, during a
celebration of Chemists Day.

Born in Balneario Camboriu (SC) and with an
undergraduate degree in Chemistry Education from the Federal
University of that state, Dias has a Doctorate in Chemistry
(UNICAMP) and a Post-Doctorate (Harvard University/EUA, 1994-1995).
He is a full professor of the Institute of Chemistry at UNICAMP and
a CNPq researcher, a full member of the Brazilian Academy of
Sciences and a Commander of the National Order of Scientific Merit.
In 2008, his laboratory was accredited by the World Health
Organization (WHO) as a World Reference Center for the synthesis of
compounds for the treatment of Chagas disease.
As acknowledgment of his professional
history, Professor Dr. Luiz Carlos Dias was asked to deliver the
commencement speech for the 38th Meeting of the Brazilian Society
of Chemistry (RASBQ), which will take place in May 2015, at Aguas
de Lindoia SP.

Prof. Luiz Carlos Dias
Aside from the Walter Borzani Award,
Professor Luiz Carlos Dias was awarded the
Santander Universities Award 2014 Edition, Science and
Innovation, in Health, with the project Optimization of lead
compounds for the treatment of tropical parasite diseases", in
cooperation with Drugs for Neglected Diseases Initiative (DNDi) and
Medicines for Malaria Venture (MMV).
The award ceremony took place on November
05, 2015, at the Hotel Grand Hyatt, in Sao Paulo, with the presence
of the President of Santander Bank Brazil, Jesus Zabalza, and of
the governor of Sao Paulo, Geraldo Alckmin, among other
authorities. The 2014 edition of “Santander Universities†awarded
the scientist with a prize in the value of R$ 100 thousand
Brazilian Reais.
HIGHLIGHTS
DOCKING, SYNTHESIS AND ANTIPROLIFERATIVE ACTIVITY OF N-ACYLHYDRAZONE DERIVATIVES DESIGNED AS COMBRETASTATINA4 ANALOGUES
PLoS ONE 9 (2014) e85380
[doi:
10.1371/journal.pone.0085380]
Daniel Nascimento do Amaral,
Bruno C. Cavalcanti, Daniel P. Bezerra, Paulo Michel P. Ferreira,
Rosane de Paula Castro, José Ricardo Sabino, Camila Maria Longo
Machado, Roger Chammas, Claudia Pessoa, Carlos M. R. Sant’Anna,
Eliezer J. Barreiro, Lídia Moreira Lima
Cancer is the second cause of death in USA.
Among the known classes of anticancer agents the
microtubule-targeted antimitotic drugs are considered one of the
most important. They are usually classified into two main groups.
One group, known as the microtubule-destabilizing agents, which
inhibits microtubule polymerization, such as the Vinca alkaloids,
vincristine (1) and vinblastine (2) – the first anti-microtubule
agents approved to treat cancer disease. The second group is known
as the microtubule-stabilizing agents that stimulate microtubule
polymerization such as paclitaxel, used to treat breast and ovarian
cancer, non-small-cell lung cancer and Kaposi’s sarcoma.
In attempts to develop
orally available anti-microtubule agents that may overcome the
neurotoxicity and the advance of resistance commonly described
for Vincaalkaloids,
paclitaxel and analogues; the combretastatin A4 (CA-4) is being
considered a promise lead-compound. This natural stilbene isolated
from Combretum caffrumbinds to the colchicine domain on β-tubulin and exhibits low toxicity
profile. However, CA-4 (4) failed to exhibit anticancer efficacy in
animal models due to its low solubility in water, lack of oral
bioavailability, short half-life and the in vivo isomerization of
double bound that implies in loss affinity for β-tubulin and
consequently loss of cytotoxic activity.
This paper the docking
study, synthesis and antiproliferative activity of
N-acylhydrazone
derivatives (5a-r)
designed as CA4 analogues are reported.
The genesis conception
of N-acylhydrazone
derivatives (5a-r)
is depicted in Figure 1. The main structural modifications was
based on the replacement of ethylene linker between the aromatic
subunits A and B by a more stable scaffold represented by
the N-acylhydrazone
(NAH) moiety, originating compound 5a. In order to design a congeneric
series (5b-r)
several modifications were introduced in the nature of aromatic
subunit B based on docking studies with colchicine binding site of
β-tubulin protein (Figure 2).
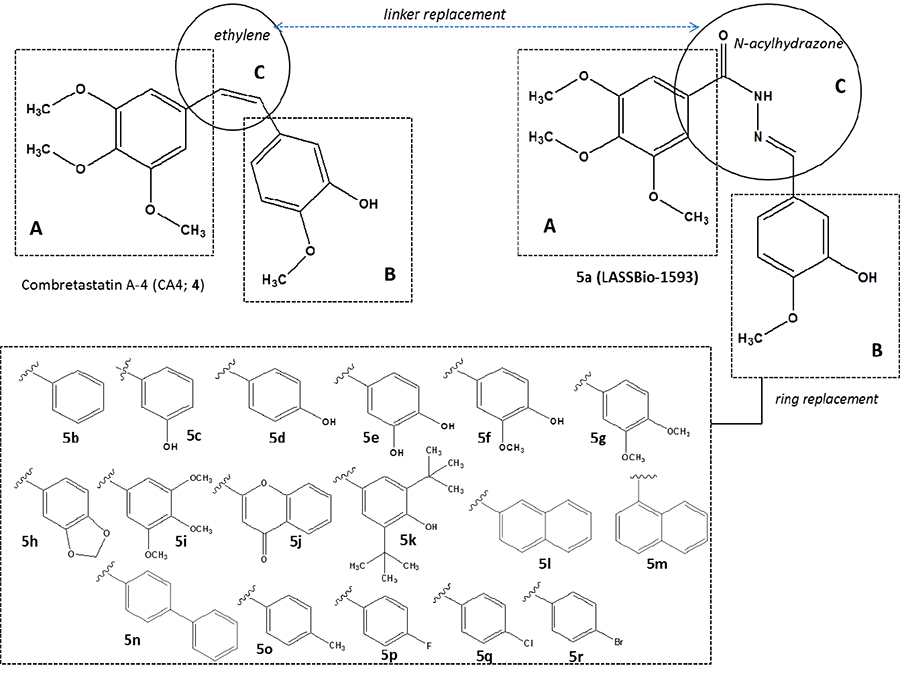
Figure
1: Initial conception and
molecular design of N-acylhydrazone derivatives 5a-r
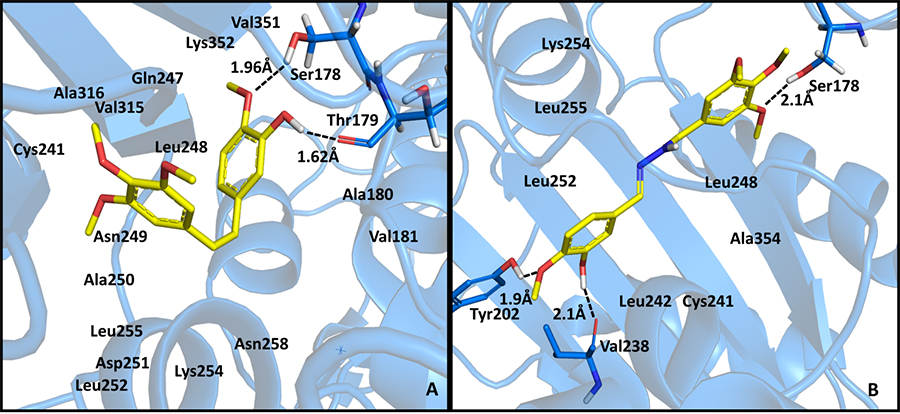
Figure
2: Polar interactions between
CA-4 (A) or LASSBio-1593 (B) with the colchicine
binding site of β-tubulin (PDB code: 1sa0). Compounds
5a-r were easily
synthetized and the characterization of imine double bond (N=CH)
was performed unequivocally using X-ray diffraction studies, as
exemplified for compound 5b (Figure 3).
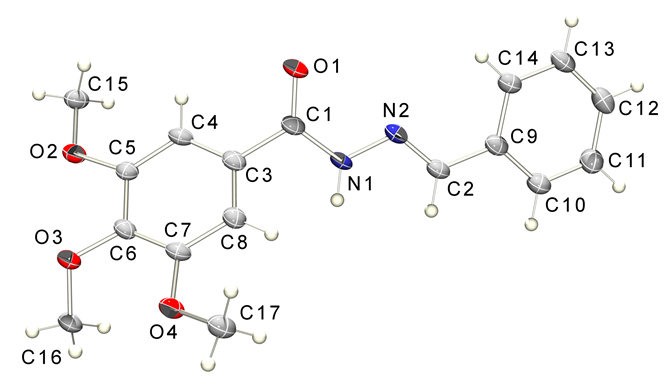
Figure
3: ORTEP view of
compound5bwith the atom displacement ellipsoids drawn at a
50% probability level.
The antiproliferative
activity of compounds 5a-r was determined based on an MTT
assay, using CA-4 as standard, against the tumor cell lines: HL-60
(human leukemia), SF-295 (human glioblastoma), MDA-MB435
(melanoma), PC3M (prostate cancer), OVCAR-8 (ovaries
adenocarcinoma), NCI-H258M (pulmonary bronchio-alveolar carcinoma)
and HCT-8 (adenocarcinoma ileocecal). To determine the selectivity
index of compounds 5a-r, their antiproliferative profile was also evaluated toward
human lymphocytes (Table 1).
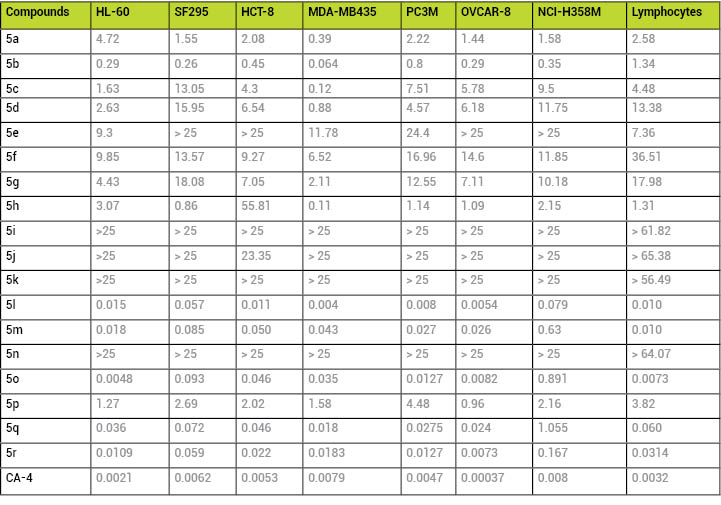
Table
1:In vitro antiproliferative potency
(IC50-•M) of
compounds 5a-rand
the standard CA-4 against tumor cell lines and human
lymphocytes.
Considering the
IC50(≤0.8•M
and ≥0.064 •M) and
the SI values, LASSBio-1586 (5b) was selected as the most
promising compound, and its ability to inhibit tubulin
polymerization was investigated. The tubulin polymerization assay
was performed by CEREP™
employing a single concentration of
5b (C = 30 µM),
using vinblastine as positive control. In this
assay, LASSBio-1586 (5b) inhibited 91% of the tubulin polymerization, validating the
rational design employed in the molecular design of the
derivatives 5a-r.
Further, the antitumor activity of LASSBio-1586 was investigated
using the Hollow Fiber Assay in BALB/c nude
mice. As shown in Table 2, LASSBio-1586 (5b; dosages = 25 and 50 mg/kg/day)
reduced the proliferation of both SF-295 (61.89 and 82.89%) and
HCT-116 (72.68 and 80.76%) cell lines after 4 days of
administration (P < 0.05), demonstrating its antiproliferative
effect in vivo.
Taken together,
LASSBio-1586 (5b)
emerged as a simple antitumor drug candidate and was capable of
inhibiting microtubule polymerization.
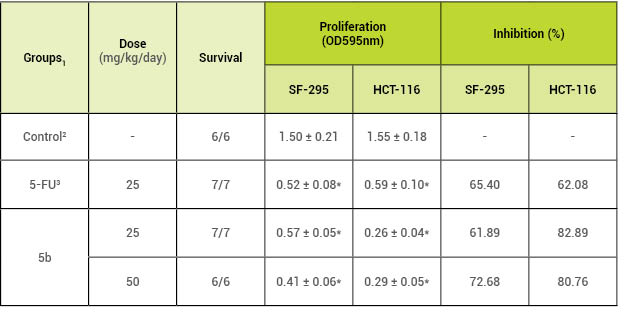
Table 2: In vivo antiproliferative activity of 5band 5-fluorouracil (5-FU) against
tumor cells as evaluated by the in Hollow Fiber Assay
(HFA).
1The data are reported as the
mean ± S.E.M., n=6-7 animals/group, which were treated for 4 days
intraperitoneally. 2The negative control group
received 5% DMSO. 35-Fluorouracil (5-FU) was
used as the positive control.*P < 0.05 compared to the
control by ANOVA, followed by Newman-Keuls test.
COMMENTS FROM
AUTHOR
A new
series of synthetic CA-4 analogues was designed based on docking
studies with β-tubulin (PDB code: 1sa0). Compounds were easily
synthetized and among them 5b (LASSBio-1586) stood
out showing cytotoxic potency varying between
IC50=
0.8•M
to 64 nM, against different
tumor cells. This compound showed better aqueous solubility than
the natural prototype (CA-4) and possessedbetter
selectivity index than CA-4. The minimum structural requirements essential for the
anti-tubulin activity of LASSBio-1586 was proposed and its
antiproliferative activity, in vivo, was
demonstrated using Hollow
Fiber Assay in BALB/c nude mice.
NOVEL
2-CHLORO-4-ANILINO-QUINAZOLINE DERIVATIVES AS EGFR AND VEGFR-2 DUAL
INHIBITORS
Eur. J. Med.
Chem.71(2014) 1-14.
[doi:10.1016/j.ejmech.2013.10.058]
Maria Letícia de Castro Barbosa,
Lídia Moreira Lima, Roberta Tesch, Carlos Mauricio R. Sant’Anna,
Frank Totzke, Michael H. G. Kubbutat, Christoph Schächtele, Stefan
A. Laufer, Eliezer J. Barreiro
Protein kinases play
important roles in the regulation of numerous cellular processes,
including proliferation, differentiation and survival. In
particular, the epidermal growth factor receptor (EGFR) and the
vascular endothelial growth factor receptor 2 (VEGFR-2) play key
roles in tumor growth and angiogenesis. EGFR and VEGFR-2 are
closely linked transmembrane receptor tyrosine kinases, sharing
common downstream signal transduction pathways. Their functional
relationship in cancer therapy is well known, i.e.inhibition of VEGFR-2 signaling
pathway contributes to the antitumoral effect of EGFR inhibitors;
whereas activation of VEGF expression independent of EGFR signaling
is thought to be one of the resistance mechanisms to anti-EGFR
therapy. The tyrosine kinases EGFR and VEGFR-2 are validated
targets in cancer therapy and several inhibitors have been approved
by the FDA for clinical use in EGFR and/or VEGFR-2 overexpressing
solid tumors, including the ATP-mimetic tyrosine kinase inhibitors
(TKIs) gefitinib (1) for EGFR and sorafenib
(4) for VEGFR-2
(Figure 1).
Secondary resistance following the initial benefits
of treatment with approved EGFR inhibitors remains a challenge in
cancer therapy and demonstrates the need for the development of
novel therapeutic alternatives. In this context, dual inhibition of
EGFR and VEGFR-2 represents a promising approach for cancer
treatment. Considering the great interest in associating EGFR and
VEGFR-2 inhibition, we have performed the design of novel dual
inhibitors of the tyrosine kinases EGFR and VEGFR-2, which are
structurally and clinically related (Figure 1).
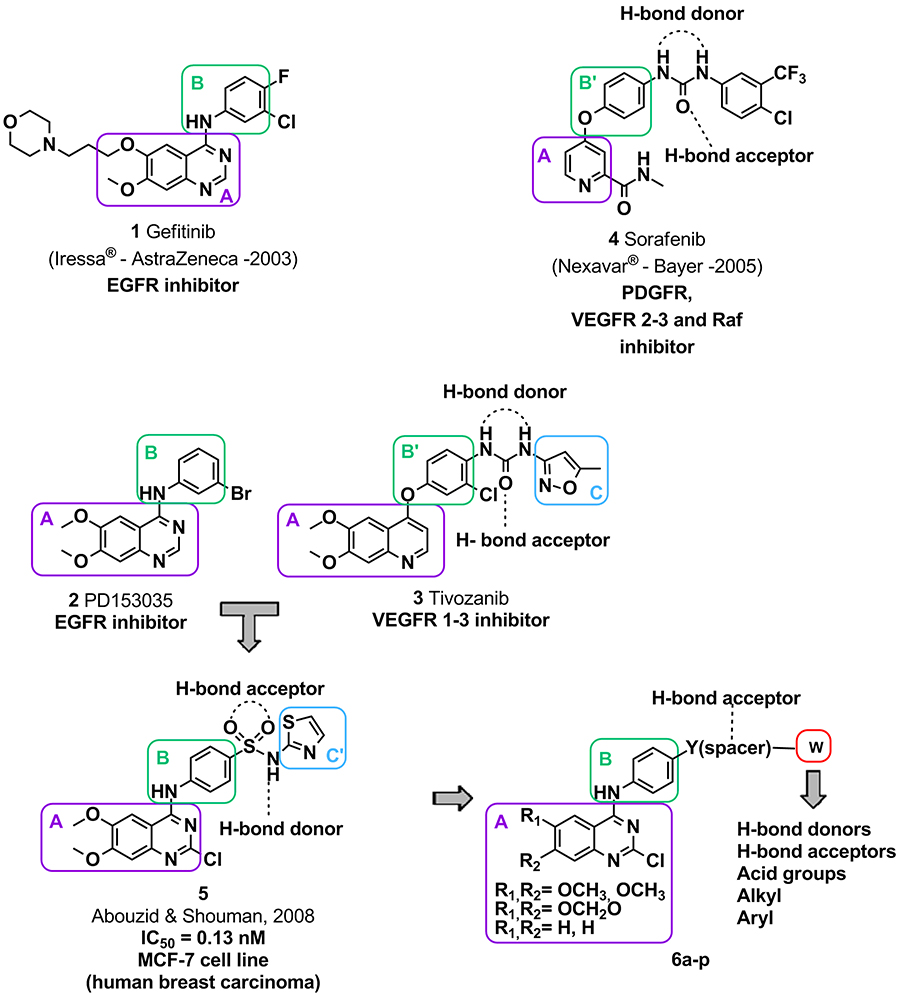
Figure 1:
Structural design of the target
2-chloro-4-anilino-quinazoline derivatives (6a-p), planned as EGFR and VEGFR-2
dual inhibitors, starting from the prototypes 1-5. Prototype 5 was previously described by Abouzid
& Shouman, Bioorganic & Medicinal Chemistry
2008,16,
7543-7551.
The designed
4-anilino-quinazoline compounds (6a-p) were synthesized by a key
condensation step between the 2,4-dichloro-quinazoline
intermediates and the corresponding aniline derivatives through
either a nucleophilic aromatic substitution or a
Buchwald-Hartwig amination (Scheme 1)
and their inhibitory activity was evaluated
employing aradiometric protein kinase assay
(33PanQinase™Activity Assay)
(Table 1).
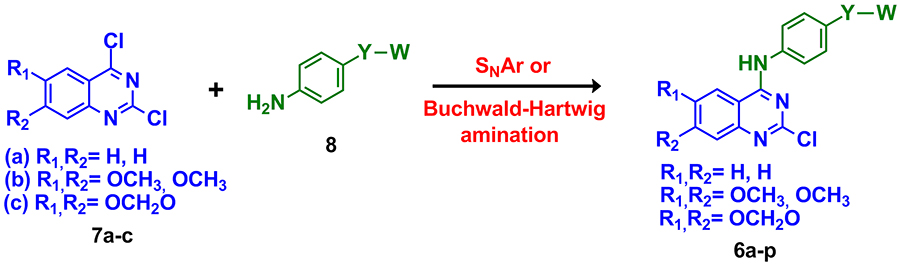
Scheme 1:
General synthesis of the designed
2-chloro-4-anilino-quinazoline compounds 6a-p. a) DIPEA, dioxane, 80°C, 12 h,
60-66%; b) isopropyl alcohol, 82°C, 24 h, 67-72%; c) ethanol, 78°C,
24 h, 64-73%; d) Pd(oAc)2, XPhos, tBuONa, tBuOH, toluene, 90°C, 1
h, 45-55%.
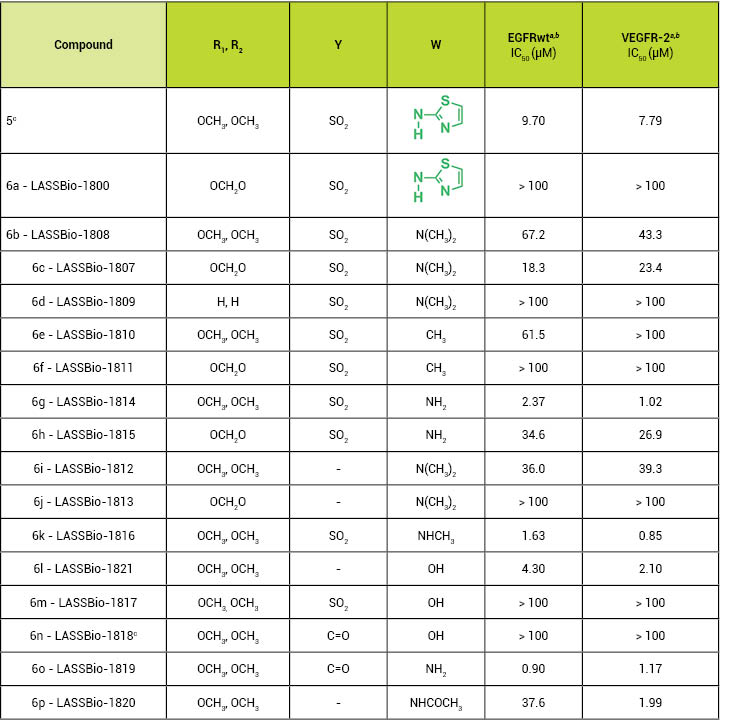
Table 1:
EGFRwt and VEGFR-2 inhibition by the
2-chloro-4-anilino-quinazoline derivatives.
aA radiometric protein kinase
assay (33PanQinase™Activity Assay) was used to
measure the kinase activity of the protein kinases EGFRwt and
VEGFR-2.b The IC50 values were calculated using
Quattro Workflow V3.1.0 (Quattro Research GmbH, Munich, Germany;
www.quattroresearch.com) and are in μM. cPreviously described by
Abouzid & Shouman, Bioorganic &
Medicinal Chemistry 2008,16, 7543-7551.
As shown in Table 1, those
derivatives containing a hydrogen bond donor at the
paraposition of the
aniline moiety presented lower IC50values, highlighting
compounds 6g (LASSBio-1814; IC50= 2.37 μM for EGFRwt and 1.02
μM for VEGFR-2), 6k (LASSBio-1816; IC50= 1.63 μM for EGFRwt and 0.85
μM for VEGFR-2) and 6o (LASSBio-1819; IC50= 0.90 μM for EGFRwt and 1.17
μM for VEGFR-2) as dual inhibitors of both the EGFR and VEGFR-2
tyrosine kinases.
Therefore, the biological
data have demonstrated the relevance of a hydrogen bond donating
substituent at the paraposition of the aniline (Figure 2) for interaction with the
EGFR and VEGFR-2 tyrosine kinase domain binding sites.
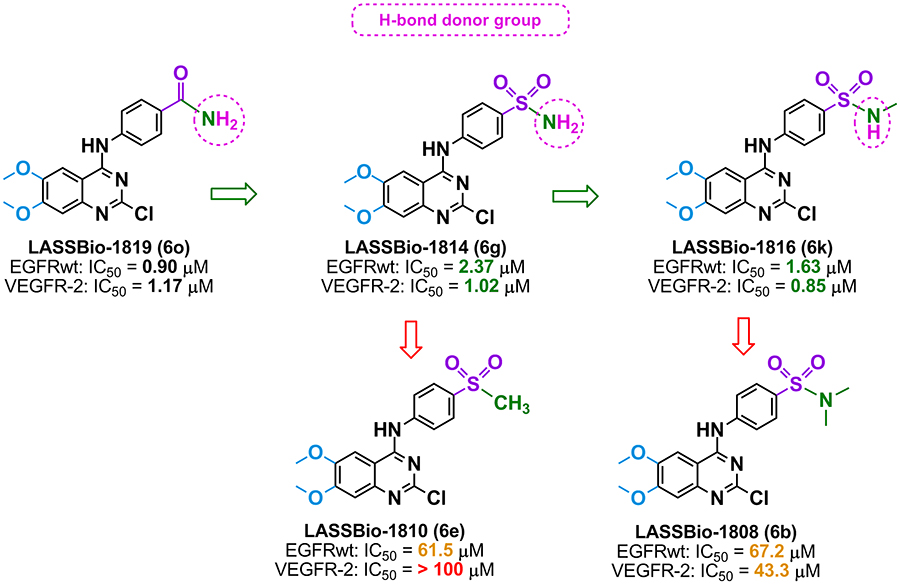
Figure 2: The relevance of a hydrogen bond donating substituent at the para position of the aniline (Table 1) for interaction with the EGFR and VEGFR-2 tyrosine kinase domain binding sites.
A docking study of this new
class of ligands with the tyrosine kinase domains of EGFRwt and
VEGFR-2 was performed to elucidate the molecular reasons behind the
observed inhibition profile, as illustrated in the Figure 3 for
compounds 6k and 6o.
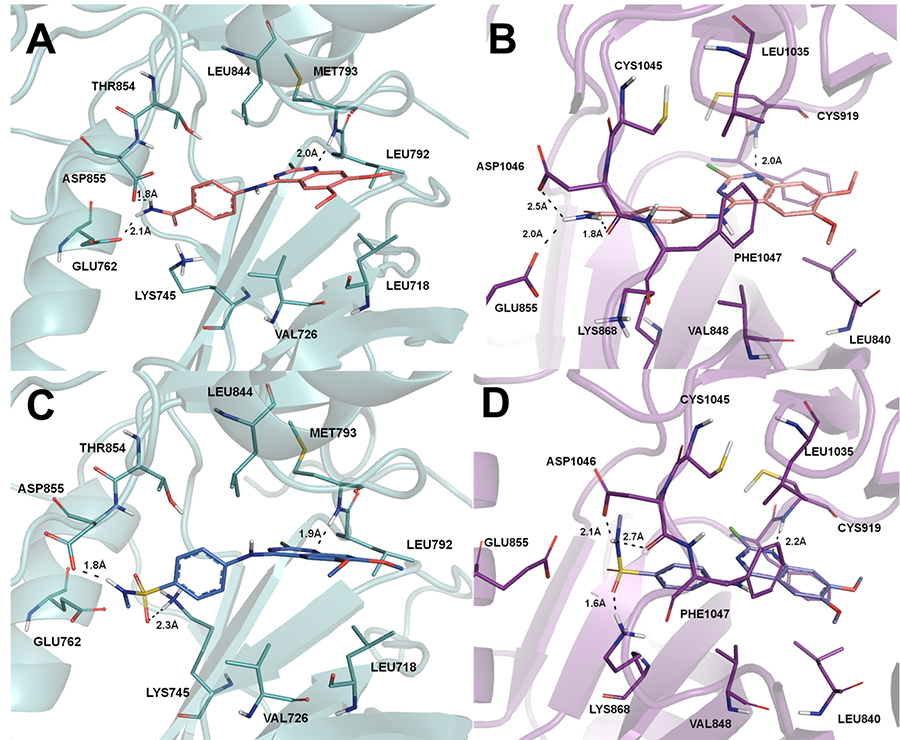
Figure
3: (A) Binding interactions of LASSBio-1819
(6o) with EGFRwt;
(B) Binding interactions of LASSBio-1819 (6o) with VEGFR-2; (C) Binding
interactions of LASSBio-1816 (6k) with EGFRwt; (D) Binding
interactions of LASSBio-1816 (6k) with VEGFR-2. Docking studies
were performed with the GOLD 5.1 program. Apolar hydrogen atoms
were omitted to improve clarity. The images were generated with
PyMol software.
In conclusion, this study
has described the synthesis and biological testing of a novel
series of 2-chloro-4-anilino-quinazoline EGFR and VEGFR-2 dual
inhibitors. The associated modulation of these two tyrosine kinases
represents a promising therapeutic approach to overcome and prevent
resistance in cancer therapy due to a synergistic effect. Moreover,
this study identified pharmacophoric groups for binding to the
selected therapeutic targets and demonstrated the importance of a
hydrogen bond donor at the paraposition of the aniline moiety for
interaction with the conserved Gluand Asp amino acids in the EGFR and
VEGFR-2 binding sites, which promotes a significant increase in
potency (Figure 4).
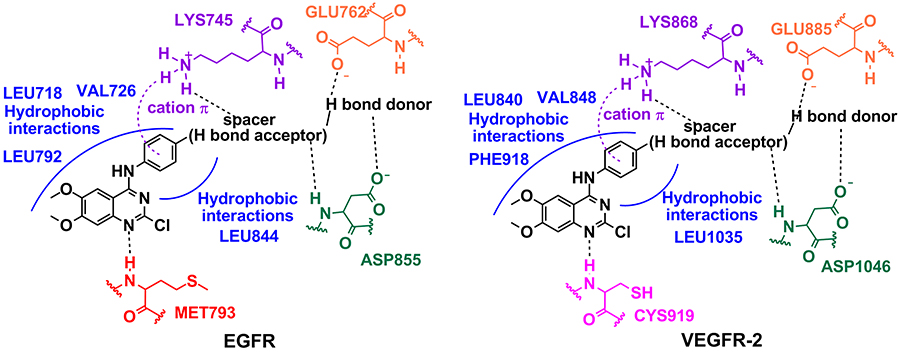
Figure
4: Pharmacophoric model for the interaction
of the 2-chloro-4-anilino-quinazoline derivatives with the
corresponding amino acid residues in the binding sites of the
selected therapeutic targets, EGFR and VEGFR-2.
COMMENTS FROM AUTHOR
Novel
2-chloro-4-anilino-quinazoline derivatives were designed,
synthesized and evaluated as EGFR and VEGFR-2 dual inhibitors,
standing out compounds 6g (LASSBio-1814), 6k(LASSBio-1816) and
6o (LASSBio-1819) as the
most potent inhibitors. Moreover, the SAR and docking studies
allowed the identification of pharmacophoric groups for both
kinases and demonstrated the importance of a hydrogen bond donor at
the paraposition of
the aniline moiety for interaction with conserved Glu and Asp amino
acids in EGFR and VEGFR-2 binding sites. These compounds present a
great potential for future investigation as antitumor drug
candidates, because EGFR and VEGFR-2 are validated targets in
cancer therapy and the combined inhibition is considered to be
synergistic for both antitumor activity and resistance
prevention.
METAL COMPLEXES WITH
2-ACETYLPYRIDINE-N(4)-ORTHO-CHLORO-PHENYLTHIOSEMICARBAZONE: CYTOTOXICITY AND EFFECT ON
THE ENZYMATIC ACTIVITY OF THIOREDOXIN REDUCTASE AND GLUTATHIONE
REDUCTASE
Eur. J. Med.
Chem. 84 (2014) 537-544 [10.1016/j.ejmech.2014.07.055]
Gabrieli L.
Parrilha, Karina S.O. Ferraz, Josane A. Lessa, Kely N de Oliveira,
Bernardo L. Rodrigues, Jonas P. Ramos, Elaine M. Souza-Fagundes,
Ingo Ott*, Heloisa Beraldo*
Cisplatin and the second
generation complexes carboplatin and oxaliplatin are antitumor
agents widely used in the treatment of a variety of solid tumors.
Despite the clinical success of cisplatin, side effects, drug resistance and treatment failure still pose
great challenges in chemotherapy with platinum complexes. Since DNA
is the primary cellular target of platinum complexes,
there is an increasing demand for novel
metal-based-pharmaceuticals with a mode of action differing from
that of the platinum generation of anticancer drugs.
Numerous metal complexes
present cytotoxic or antitumor activities, such as gallium(III),
gold(I,III), antimony(III), bismuth(III) and ruthenium(II)
complexes. Much effort is presently directed to the search for the
mechanism of action of these non-platinum compounds and of their preferential protein
targets.
Thioredoxin reductase
(TrxR) is a homodimeric selenoenzyme, which is responsible for the nicotinamide adenine dinucleotide
phosphate (NADPH)-dependent reduction of its substrate thioredoxin
(Trx) in the thioredoxin system
and for the reduction of many other oxidized cell
constituents. It is involved in several metabolic pathways and
pathophysiological conditions (cancer, infectious diseases,
rheumatoid arthritis, etc). Cancer cells
often overexpress both Trx and TrxR indicating that the thioredoxin
system may have a crucial role in tumor progression. Hence, both
Trx and TrxR might be considered as emerging targets for the
development of new anticancer drug candidates.
It has been shown that the anti-rheumatic
gold(I) complex auranofin presents antitumor activity and inhibits
TrxR with great selectivity (approximately 1000-fold) compared to
the related enzymes glutathione reductase (GR) and glutathione
peroxidase (GP). In addition, it has been proposed that the
relevant cytotoxic actions exhibited by a variety of gold(I) and
gold(III) compounds are mainly the result of potent TrxR
inhibition, suggesting that the main target of gold complexes is
TrxR. Moreover, TrxR inhibition has also been observed with metal
complexes different from gold.
Thiosemicarbazones have shown significant antineoplastic
activity against a large number of human tumor cell
lineages. α(N)-heterocyclic
thiosemicarbazones have been extensively investigated for their
anticancer activity, which has been attributed to the inhibition of
ribonucleoside diphosphate reductase (RDR), an essential enzyme
involved in the conversion of ribonucleotides into
deoxyribonucleotides during DNA synthesis. We demonstrated that
gallium(III), platinum(II), palladium(II), gold(I), antimony(III),
and tin(IV) complexes with thiosemicarbazones show cytotoxic
activity against human tumor cells. The mode of action of the
gallium(III) complexes might involve inhibition of RDR while the
palladium(II) and platinum(II) complexes probably bind to DNA and
the gold(I) complexes act as TrxR inhibitors in vitro.
In
previous works we reported that 2-acetylpyridine N(4)-ortho-, N(4)-metaand
N(4)-para-chlorophenyl thiosemicarbazone were cytotoxic at nanomolar
doses against glioma cells and were able to induce cell death by
apoptosis induction. In addition, the thiosemicarbazones also
proved to be cytotoxic at nanomolar doses against MCF-7 breast
adenocarcinoma cells, the ortho-chloro derivative being particularly
effective.
We
now prepared gold(III), platinum(II), palladium(II), bismuth(III),
tin(IV), antimony(III) and gallium(III) complexes with
N(4)-ortho-chlorophenyl-2-acetylpyridine thiosemicarbazone
(H2Ac4oClPh) (Fig. 1)
and assayed the compounds for their cytotoxic activity against
MCF-7 breast adenocarcinoma and HT-29 colon carcinoma cells. The
ability of the compounds to act as inhibitors of the enzymatic
activities of TrxR and GR was investigated.
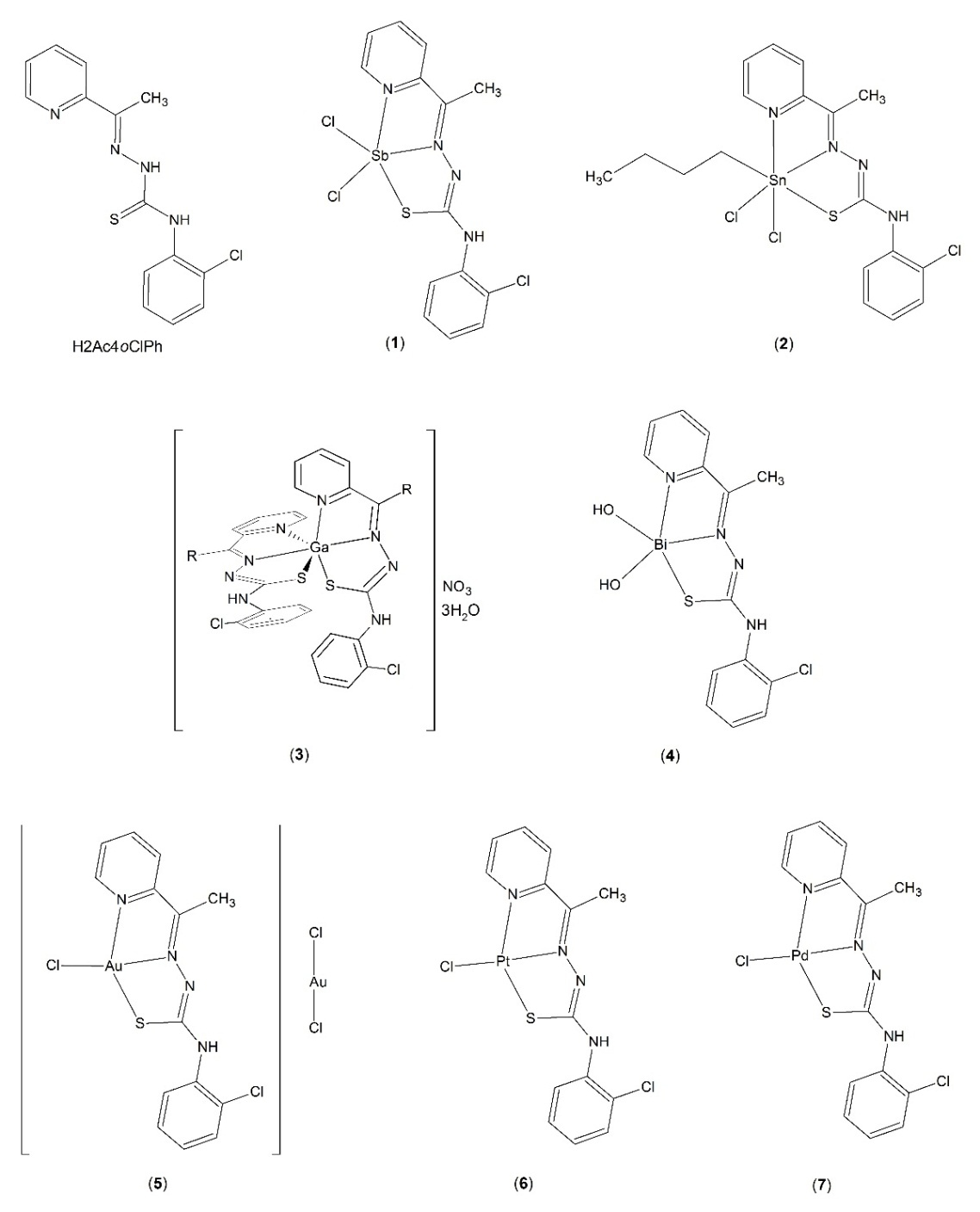
Figure
1:Structures of
2-acetylpyridine-N(4)-orthochlorophenyl thiosemicarbazone (H2Ac4oClPh) and its complexes
[Sb(2Ac4oClPh)Cl2] (1), [(n-Bu)Sn(2Ac4oClPh)Cl2] (2), [Ga(2Ac4oClPh)2]NO3·3H2O (3), [Bi(2Ac4oClPh)(OH)2] (4), [Au(2Ac4oClPh)Cl]AuCl2(5),
[Pt(2Ac4oClPh)Cl]
(6) and
[Pd(2Ac4oClPh)Cl]
(7).
H2Ac4oClPh and its antimony(III) (Figure
2), tin(IV), gallium(III), bismuth(III) and gold(III) complexes
proved to be highly cytotoxic to MCF-7 and HT29 cells whereas
the palladium(II) and platinum(II) complexes were not as effective
(Table 1). Most of the compounds under study were less cytotoxic to
non-malignant Vero cells than to the assayed tumor cell
lineages.
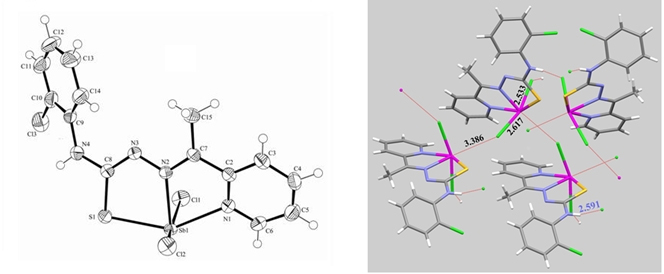
Figure 2: Ortep drawing and molecular packing of [Sb(2Ac4oClPh)Cl2] (1).
H2Ac4oClPh and its gallium(III) and tin(IV)
complexes did not show any inhibitory activity against TrxR and GR.
The palladium(II), platinum(II) and bismuth(III) complexes
inhibited TrxR at micromolar concentrations but not GR. The
antimony(III) (1)
and gold(III) (5)
complexes strongly inhibited TrxR at submicromolar doses with GR
inhibition at higher concentrations (Table 1, Figure 3). The
selectivity of these complexes for TrxR suggests metal binding to a
selenol residue in the active site of the enzyme. TrxR inhibition
is likely a contributing factor to the mode of action of the gold
and antimony derivatives.
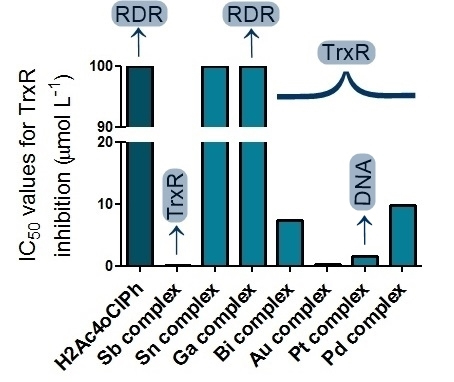
Figure
3: IC50values for TrxR inhibition by
H2Ac4oClPh and its
metal complexes.
RDR is believed to be the
main target of the thiosemicarbazone and of its gallium(III)
complexes. Since gallium(III) and iron(III) show very similar
charge-to-radius ratio, the chemical behavior of gallium(III)
closely resembles that of iron(III). Due to competitive binding of
gallium(III) and iron(III), gallium interacts directly with RDR,
displacing iron from the enzyme. Although it has been suggested
that the antiproliferative effects of organotin(IV) compounds are
related to metal binding to thiol groups of proteins, the mode of
cytotoxic action of these compounds remains largely unknown. In the
present work [(n-Bu)Sn(2Ac4oClPh)Cl2] (2) was unable to inhibit both
TrxR and GR enzymatic activities under the experimental
conditions.
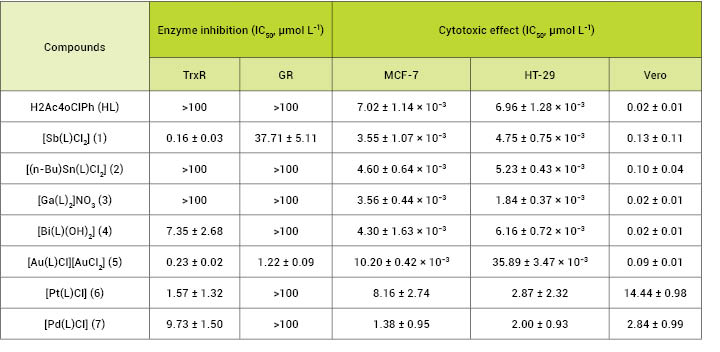
Table 1: IC50values of compounds for TrxR
and GR inhibition and cytotoxic activity against HT-29, MCF-7 and
Vero cells
Overall the cytotoxic effect
of the compounds is largely the result of the thiosemicarbazone
ligand. However, selective inhibition of TrxR by complexes
(1) and
(5) adds another
mechanism contributing to their pharmacological profile. The
complexes might thus provide prototypes for multi-target anticancer
metal-based drugs. Further structural optimization of the compounds
and elucidation of relevant cellular pathways are surely of
interest.
COMMENTS FROM
AUTHOR
DNA is the primary cellular
target of cisplatin and second generation platinum complexes.
Current research aiming to overcome the problems associated with
platinum anticancer drugs has focused on other metal-based
therapeutics with different mechanisms of action. Gold complexes
proved to have cytotoxic activity and are recognized as extremely
potent inhibitors of thioredoxin reductase (TrxR), a large
homodimeric selenoenzyme, which controls the redox state of
thioredoxin (Trx) in the thioredoxin system. In previous works we
demonstrated that 2-acetylpyridine-N(4)-ortho-chlorophenylthiosemicarbazone
(H2Ac4oClPh)
presents cytotoxic effect at nanomolar doses against human tumor
cell lineages. In the present study we showed that
H2Ac4oClPh and its
gold(III), gallium(III), tin(IV), antimony(III), and bismuth(III)
complexes are highly cytotoxic against MCF-7 breast adenocarcinoma
and HT-29 colon carcinoma cells. The antimony(III) and gold(III)
complexes strongly inhibited TrxR at submicromolar doses with GR
inhibition at higher concentrations. The selectivity of these
complexes for TrxR suggests metal binding to a selenol residue in
the active site of the enzyme. Selective inhibition of TrxR by the
antimony(III) and gold(III) complexes adds another mode of action
contributing to their pharmacological profile. As we mentioned in
the published article, the identification of TrxR as a target for
gold(III) and antimony(III) complexes might hopefully lead to the
discovery of more effective, “mechanism-oriented”, anticancer
metal-based drugs. In addition, to our knowledge this is the first
report on inhibition of TrxR by an antimony(III) compound. This
finding is important also due to the known anti-parasitic effects
of antimonial drugs since TrxR could be an additional target for
their pharmacological or toxic effects.
N-ACYLHYDRAZONE DERIVATIVE AMELIORATES MONOCROTALINE-INDUCED
PULMONARY HYPERTENSION THROUGH THE MODULATION OF ADENOSINE AA2R
ACTIVITY
International J.
Cardiol. 173(2014)
154-162 [doi.org/10.1016/j.ijcard.2014.02.022]
Allan K.N. Alencar,
Sharlene L. Pereira, Flavia E. da Silva, Luiza V.P. Mendes, Valéria
do M.N. Cunha, Lidia M. Lima, Tadeu L. Montagnoli, Celso
Caruso-Neves, Emanuelle B. Ferraz, Roberta Tesch, José H.M.
Nascimento, Carlos M.R. Sant'Anna, Carlos A.M. Fraga, Eliezer J.
Barreiro, Roberto T. Sudo, Gisele Zapata-Sudo
Pulmonary arterial hypertension (PAH) is
characterized by excessive pulmonary vasoconstriction and abnormal
vascular remodeling processes that usually affect all vessel layers
and result in severe loss of cross-sectional area and, therefore,
increased right ventricular (RV) afterload. Although the
pathogenesis of PAH is incompletely understood, evidence suggests
that PAH is associated with activation of inflammatory processes,
endothelial damage and dysfunction, and abnormal coagulation.
Adenosine
is a potent modulator of cardiovascular function. Adenosine
A2Areceptors
(A2AR) are located
primarily in the vasculature where they mediate vasodilatation, and
in the heart they promote cardioprotective effects. Current
therapies for chronic PAH are designed to reduce pulmonary arterial
resistance by inducing vasodilatation, but these therapies only
provide symptomatic relief. Recently, we have shown that an
N-acylhydrazone derivative from safrole, a substance present in
sassafras oil, may contribute to the prevention of MCT-induced PAH
by reversing pulmonary vascular remodeling, which in turn reduces
RV hypertrophy.
In the
present study, we investigated the efficacy and a possible
molecular mechanism of (E)-N'-(3,4dimethoxybenzylidene)-4-methoxybenzohydrazide
(LASSBio-1386), a new compound of the N-acylhydrazone class
synthesized by our group (Fig. 1A), in
MCT-induced PAH rats. The vasodilator activity of LASSBio-1386 was
evaluated in pulmonary artery rings from normal Wistar rats. The
compound induced relaxation of Phe-contracted vessels
(10−5
M) in a concentrationdependent
manner. The concentration of LASSBio-1386 that reduced 50% of the
Phe-induced contraction (IC50) was 6.8 ±
0.6 μM
(Fig. 1B). The vasodilator effect of LASSBio-1386 was
investigated in the presence of ZM 241385
(10−7
M), which is a selective antagonist
of A2AR.
Pretreatment of pulmonary arteries with ZM 241385 induced a
rightward shift of the concentration-response curve and reduced the
maximal relaxation from 100% to 57.4% ± 1.8% (P b 0.05,
Fig. 1B). The proposed molecular rationale for the activation of
A2AR by
LASSBio-1386 was determined observing the highest score pose
obtained after a docking run into the binding site of
A2AR (PDB ID
3EML). It can be observed that the methoxy group of the
para-methoxyphenyl subunit of LASSBio-1386 makes a hydrogen bond
with the peptidic NH group of this same residue, but this
interaction is expected to be weaker than the hydrogen bond of ZM
241385 that involves the negatively charged carboxylate group of
Glu169 (Fg.1C-D).
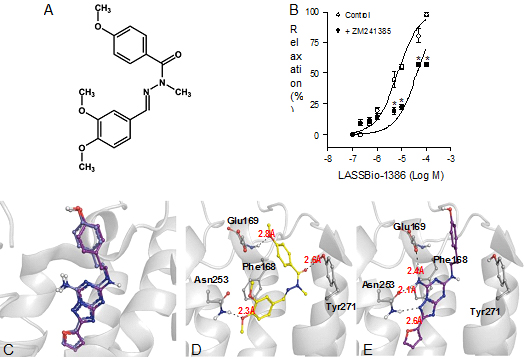
Figure
1:(A) Chemical structure of
(E)-N'-(3,4-dimethoxybenzylidene)-4-methoxybenzohydrazide
(LASSBio-1386). (B) Concentration-response curves for LASSBio-1386
in pulmonary artery rings from normal Wistar rats, contracted with
phenylephrine (10−5 mol/L), in the
presence or absence of ZM 241385 (10−7 mol/L). Data are mean ± SEM (n = 5). *P b 0.05 compared to
control. (C) Superposition of ZM241385 conformation in the crystal
structure of A2A receptor (purple) and
that obtained after re-docking (light purple) using the program
GOLD 5.2. RMSD = 0.63 Å. (D) Binding mode predicted of LASSBio-1386
and its interactions in A2A adenosine receptor.
(E) Interactions of the co-crystallized antagonist ZM 241385 in the
A2A adenosine receptor
(PDB ID: 3EML).
The animals were submitted
to a treadmill test before, 14 days after, and 28 days after the
MCT injection. The EC for control rats before the MCT injection was
1631.0 ± 67.5 m·kg, and for the MCT groups (MCT, MCT + vehicle, and
MCT + LASSBio-1386) it was 1715.0 ± 52.7 m·kg, 1677.0 ± 78.5 m·kg,
and 1812.0 ± 49.4 m·kg, respectively. Fourteen days after the MCT
injection, the EC was significantly reduced from 1544.0 ± 109.1
m·kg in the control group to 871.7 ± 27.8 m·kg (MCT) and 760.9 ±
34.7 m·kg (MCT + vehicle). Fourteen days after MCT administration
in animals that were treated with LASSBio-1386 (MCT +
LASSBio-1386), the EC was further reduced to 630.1 ± 31.2 m·kg (P b
0.05 vs. control); while at 28 days after MCT injection, oral
treatment with LASSBio-1386 significantly increased the EC to
1357.0 ± 87.8 m·kg (Fig.
2).
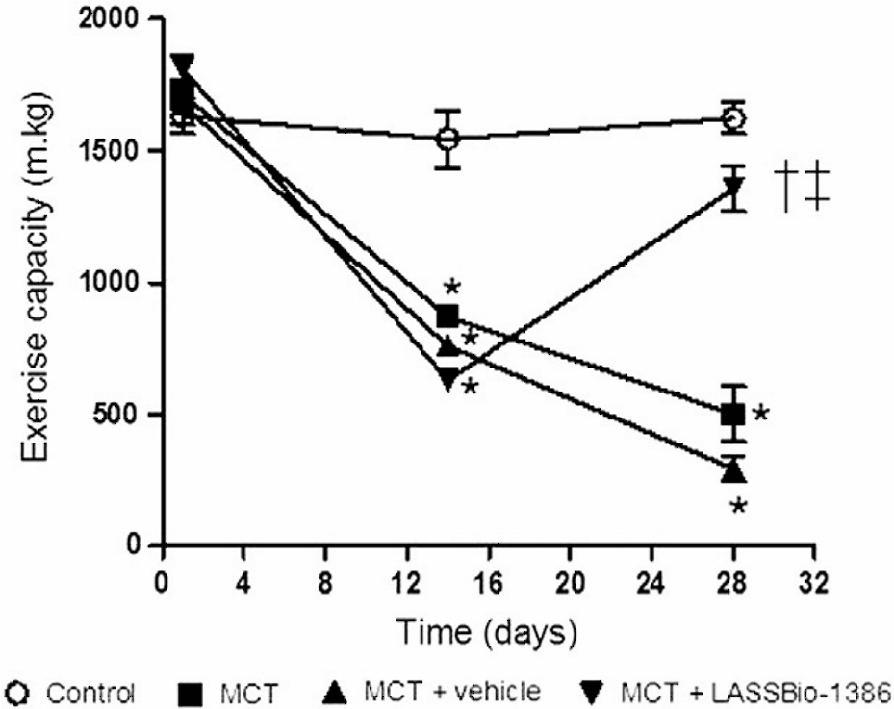
Figure
2:Effects of the oral treatment with
vehicle (DMSO) or LASSBio-1386 (50 mg/kg/day) of MCT-injected rats
in exercise test protocol. Data are mean ± SEM (n =5-6). *P b 0.05
compared to control; †P b 0.05 compared to MCT; ‡P b 0.05 compared
to MCT + vehicle. All groups were evaluated before MCT injection,
14 and 28 days after MCT injection. 14 days after MCT injection,
MCT-injected rats received vehicle or LASSBio-1386 for 2
weeks.
Pulmonary hypertension and
RV dysfunction were found in MCT-treated rats as indicated by a
significant increase in RVSP values at day 28, compared with the
control rats (49.60 ± 5.0 mm Hg vs. 27.28 ± 2.1 mm Hg, P b 0.05).
However, RVSP was attenuated in rats treated with LASSBio-1386 at a
dose of 50 mg/kg (27.03 ± 1.2 mm Hg) (Fig.
3).
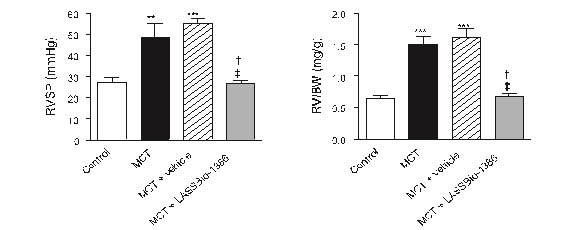
Figure 3:
Effects of the oral treatment with LASSBio-1386
(50 mg/kg/day) for 2 weeks on right ventricular systolic pressure
(RVSP) and on right ventricular (RV) hypertrophy in MCT-injected
rats. (A) Representative tracings of RVSP of control rats,
monocrotaline (MCT), MCT + vehicle (DMSO), and MCT + LASSBio-1386,
respectively. (B) Right ventricular systolic pressure (RVSP). Oral
treatment with LASSBio-1386 recovered this parameter. (C) RV weight
to body weight ratio [RV/BW]. Treatment with LASSBio-1386 decreased
the RV hypertrophy. Each column represents the mean ± SEM (n = 6).
**P b 0.01, ***P b 0.001 compared to control; †P b 0.05 compared to
MCT; ‡P b 0.05 compared to MCT + vehicle.
Representative images of
the pulmonary arterioles are shown in Fig.
4. The wall thickness of the pulmonary
arterioles (b50 μm) was significantly increased from 64.7% ± 1.7%
(control rats) to 77.2% ± 2.6% (MCT + vehicle rats). Oral treatment
with LASSBio-1386 (50 mg/kg) reduced the wall thickness of these
vessels to 69.1% ± 1.6% (P b 0.05 vs. MCT + vehicle;
Fig. 4). In vessels with
diameter ranging between 50 and 150 μm, the wall thickness was
increased from 56.2% ± 2.3% (control rats) to 66.9% ± 2.3% (MCT +
vehicle rats). The wall thickness of pulmonary arterioles of
MCT-injected rats treated with LASSBio-1386 decreased to 57.9% ±
1.8% (P b 0.05 vs. MCT + vehicle; Fig.
4).Western blot analysis of RV tissue
showed that PAH reduced A2AR expression
(Fig. 5).
LASSBio-1386 enhanced the levels of A2AR in the RV from MCT-induced pulmonary hypertensive rats. To
determine which molecular pathways are involved in LASSBio1386
intervention in RV dysfunction, we investigated the effects of
LASSBio-1386 on Ca2+ handling through
SERCA2a and PLB expression. SERCA2a protein expression was
downregulated, while PLB was overexpressed in PAH rats. After
treatment with LASSBio-1386, SERCA2a protein expression was
elevated, and a reduction in the PLB protein level was observed.
PAH induced a reduction in Ca2+-ATPase activity in RV tissues. However, LASSBio-1386 was
found to reverse this reduction of Ca2+-ATPase activity in MCT-treated rats.
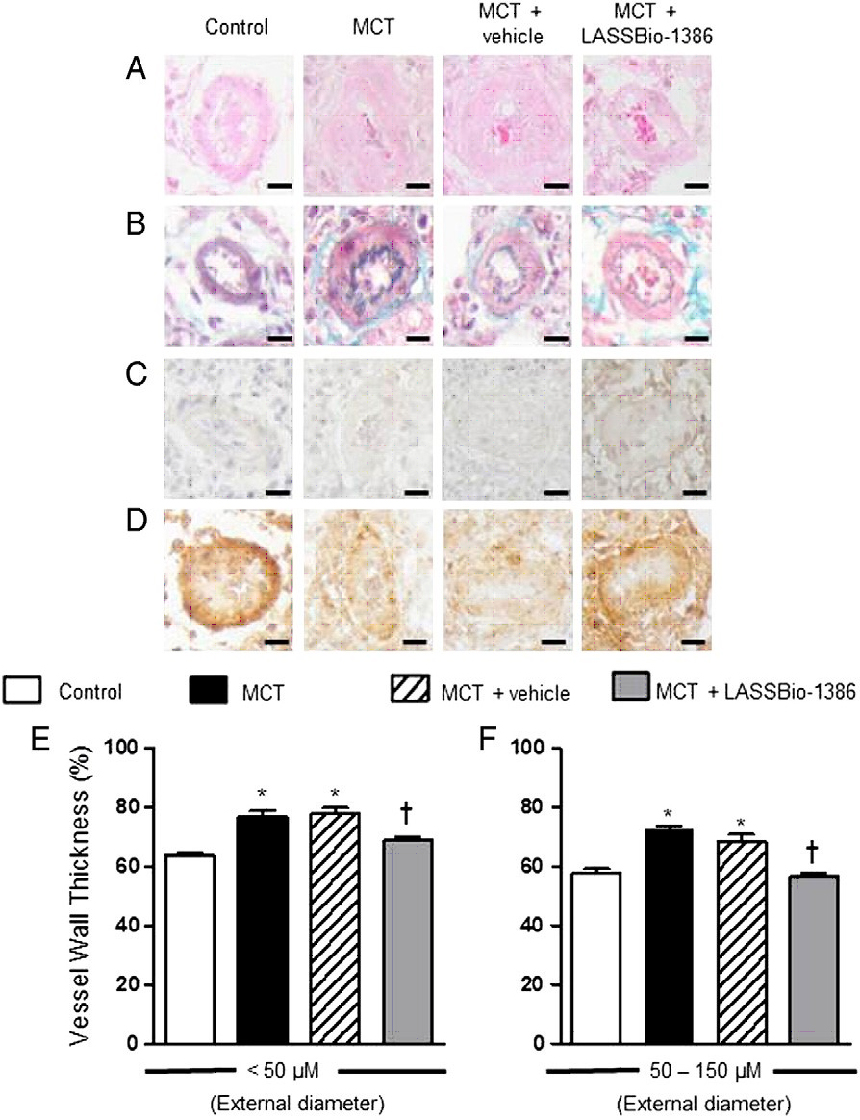
Figure 4: Representative images of lung sections of control rats and MCT-injected rats treated orally with vehicle (DMSO) or LASSBio-1386 (50 mg/kg/day). Images show vessels at 40x magnification. Each bar represents 20 µm. (A) Hematoxylin and Eosin; (B) Gomori’s trichrome; (C) Negative control and (D) Immunohistochemical staining for alpha-actin. (E) Wall thickness expressed as a percent of the total area of the vessel (b 50 μm). (F) Wall thickness of vessels ranging between 50 – 150 μm in external diameter. Each column represents the mean ± SEM.
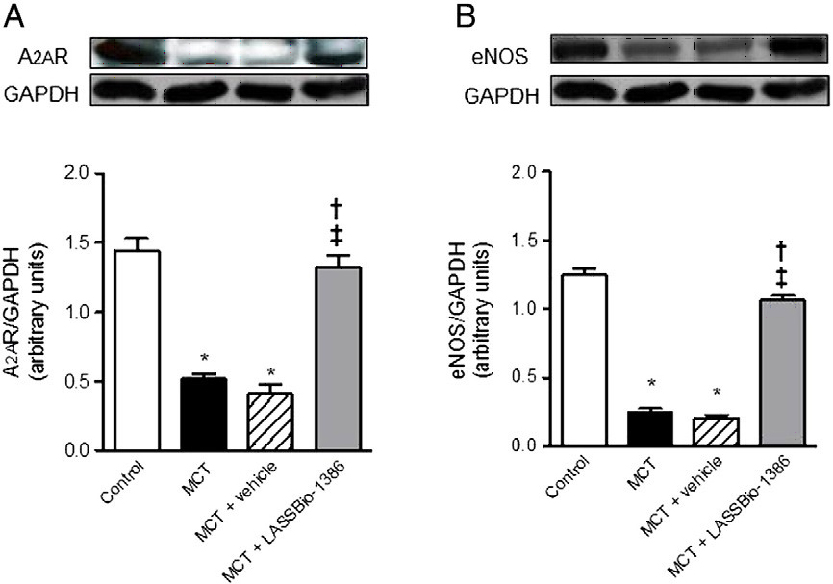
Figure 5: Western blot analyses of (A) adenosine A2A receptor (A2AR) and (B) endothelial NO synthase (eNOS) expression in lungs from control, monocrotaline (MCT), MCT + vehicle (DMSO), and MCT + LASSBio-1386 groups, respectively. GAPDH was used for normalization. Graphs show protein quantification. Each column represents the mean ± SEM (n = 5-6).
The present study shows
that LASSBio-1386 reduces pulmonary vascular remodeling, RV
systolic pressure, and RV hypertrophy in rats with MCT-induced PAH.
Moreover, we were able to demonstrate that these beneficial effects
are accompanied by a significant improvement of exercise capacity.
LASSBio-1386 administration decreased the presence of proliferative
changes in the pulmonary arterioles and the pulmonary vascular
remodeling as well as recovered endothelial dysfunction of
pulmonary artery rings, as assessed by the normalized ACh-induced
relaxation. This result probably occurs because
A2AR activation
represents an important regulatory mechanism to control the
development of PAH and pulmonary vascular remodeling. Pretreatment
of pulmonary artery rings with the A2AR antagonist ZM 241385 significantly decreased the
vasodilator effect of LASSBio-1386. This finding suggests the
involvement of A2AR in this
process.
COMMENTS FROM
AUTHOR
In this study, the orally administered
LASSBio-1386 reduced the hypertrophic vascular and cardiac
remodeling, which is observed in PAH. Our findings have important
pharmacological and clinical implications, as some alterations of
eNOS, A2AR, SERCA2a and PLB expression were restored after
treatment with LASSBio-1386 suggesting a promise therapeutic
approach for the disease.
MYD88-, BUT
NOT NOD1- AND/OR NOD2-DEFICIENT MICE, SHOW INCREASED SUSCEPTIBILITY
TO POLYMICROBIAL SEPSIS DUE TO IMPAIRED LOCAL INFLAMMATORY
RESPONSE
Plos
One9(8):
e103734(2014).
[DOI]
10.1371/journal.pone.0103734
Fabiane Sônego, Fernanda
V. S. Castanheira, Paula G. Czaikoski, Alexandre Kanashiro,
Fabricio O. Souto, Rafael O. França, Daniele C. Nascimento,
Andressa Freitas, Fernando Spiller, Larissa D. Cunha, Dario S.
Zamboni, José C. Alves-Filho, Fernando Q. Cunha
Sepsis is a complex syndrome
caused by the inability of the host to control an infection,
usually triggered by bacteria. Neutrophil recruitment to the
infection site has been demonstrated to be essential for the
bacterial clearance, preventing the spread of the infection. The
recruitment of neutrophils is enabled by the establishment of
inflammatory response after the pathogen detection by two main
families of pattern recognition receptors in the host immune cell:
Toll-like receptors (TLR) and Nucleotide-binding oligomerization
domain (Nod)-like receptors (NLR). In this study, we investigated
the role of Nod1, Nod2, and MyD88, the adaptor protein of the most
of TLR in the chemokine production and neutrophil recruitment after
caecal ligation and puncture (CLP) in C57Bl/6 mice.
Nod1 and Nod2
have been described to play role in the immune response to several
bacterial infections. Moreover, Nod1 ligands have been show to
induce production of chemokines and neutrophil recruitment
in vivo. Unexpectedly, neutrophil recruitment and chemokines CXCL1
and CXCL2 levels were similar in WT, Nod1- and
Nod2-deficient mice 6 and 24 h after CLP (Figure 1a, 1b, and
1c). Consequently, bacterial loads in the peritoneal cavity and the
blood were also similar in all the groups tested (Figures 1d and
1e). In addition, we demonstrated that systemic parameters such as
IL-6 and neutrophil sequestration at the lungs were not altered by
the absence of Nod1 or Nod2 (Figure 1f and 1g). As expected,
WT, Nod1- and Nod2-deficient mice
showed similar survival rates in CLP-induced sepsis (Figure 1h).
Reaffirming these results, double deficient mice for
Nod1and Nod2(Nod1/Nod2) as well as
mice lacking their downstream adaptor protein Rip2 also showed
unaltered local and systemic responses to WT mice. Neutrophil
recruitment, CXCL1, and CXCL2 local production, bacterial load in
the peritoneal cavity and blood, IL-6 systemic production and
neutrophil sequestration at the lung were similar to the ones in WT
mice (Figure 2a-g). As consequence, the survival curve was also
similar between Nod1/Nod2 and Rip2 and WT (Figure 2h and
2i).
On the other
hand, our group has reported that TLR2, 4 and 9 play deleterious
role in neutrophil recruitment and in the outcome to CLP-induced
sepsis. It is believed that the activation of many TLRs during the
polymicrobial challenge contributes to the overwhelming of
inflammatory response observed in sepsis and may leads to high
mortality rates. However, here we demonstrate that the abrogation
of most TLR signalling, assessed by MyD88-deficient mice,
leads to high susceptibility to sepsis because of the inability to
establish a local inflammatory response. Neutrophil recruitment and
CXCL1 and CXCL2 local levels were markedly reduced in
MyD88- deficient mice (Figure 3a-c) leading to increase in
bacterial load in the peritoneal cavity and in the blood (Figure 3d
and 3e). The MyD88-deficient mice
also showed a strong reduction in IL-6 levels (Figure 3f) in the
peritoneal cavity. However, MyD88-deficient
neutrophils are recruitedin
response to CXCL2 in
vitroand in vivoin
non-septic mice (Figures 4a e 4b), indicating that the reduction in
the neutrophil recruitment in MyD88-deficient mice
is not due to a reduction in the chemotactic ability of the
neutrophils. Tracked neutrophils from WT andMyD88-deficient
mice injected into WT mice 2 h before sepsis induction showed
similar numbers of WT and MyD88-deficient
neutrophils in the peritoneal lavage of WT mice after sepsis
induction (Figure 4c). Moreover, the adoptive transfer of WT
resident cells into MyD88-deficient mice before the induction of
sepsis resulted in the increase of local levels of CXCL2 (Figure
4d). Additionally, there was an increase in neutrophil recruitment
to the peritoneal cavity during CLP-induced sepsis (Figure 4e). As
expected, we showed that MyD88-deficient mice
were markedly susceptible to CLP-induced sepsis (Figure
4f).
In conclusion, our data
indicate that Nod1 and Nod2 are not required for the development of
the inflammatory response and the outcome of polymicrobial sepsis
in our experimental conditions. Nonetheless, we demonstrated that
MyD88-dependent signalling is crucial for sepsis because the
removal of this molecule completely dampened the establishment of
the local inflammatory response, culminating in a high
susceptibility to CLP-induced sepsis.
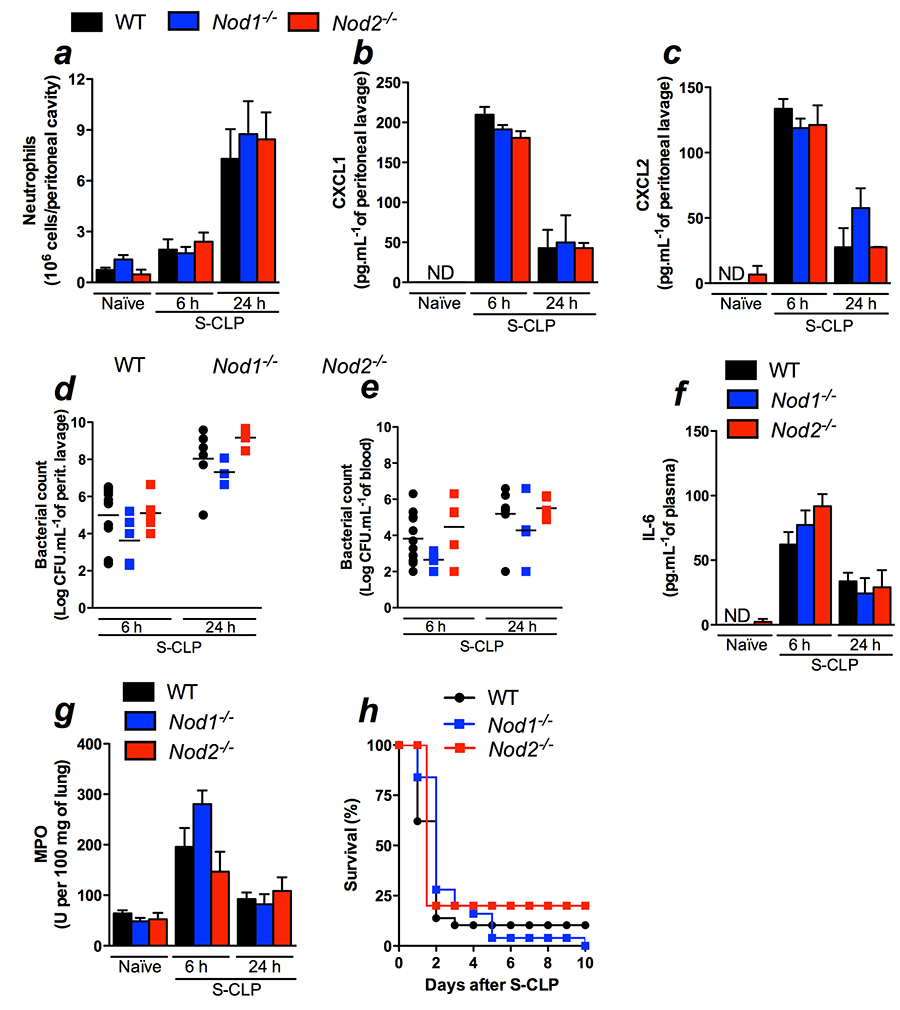
Figure
1:Nod1 and Nod2 are not
crucial for the inflammatory response during severe polymicrobial
sepsis. Six or
24h Nod1-and Nod2-deficient mice
(WT, Nod1−/−and
Nod2−/−,
respectively) underwent CLP-induced severe sepsis they were
assessed for: a) neutrophil recruitment to the peritoneal cavity;
b) CXCL1 and c) CXCL2 levels in the peritoneal lavage, as measured
by ELISA; d) bacterial count in the peritoneal lavage and e) blood;
f) IL-6 levels in plasma; g) lung MPO activity. The data were
analysed by multifactorial ANOVA and are expressed as the mean ±
SEM in a, b, c, f and g and as median in d and e. h) The survival
curve was observed up to 10 days after the induction of severe
sepsis (S). The results are expressed as percentage of survival and
were analysed by the Mantel-Cox log-rank test. The graphs represent
the mean of the results of two or three independent experiments. n
= 3 to 6 per experiment; ND = not detected.
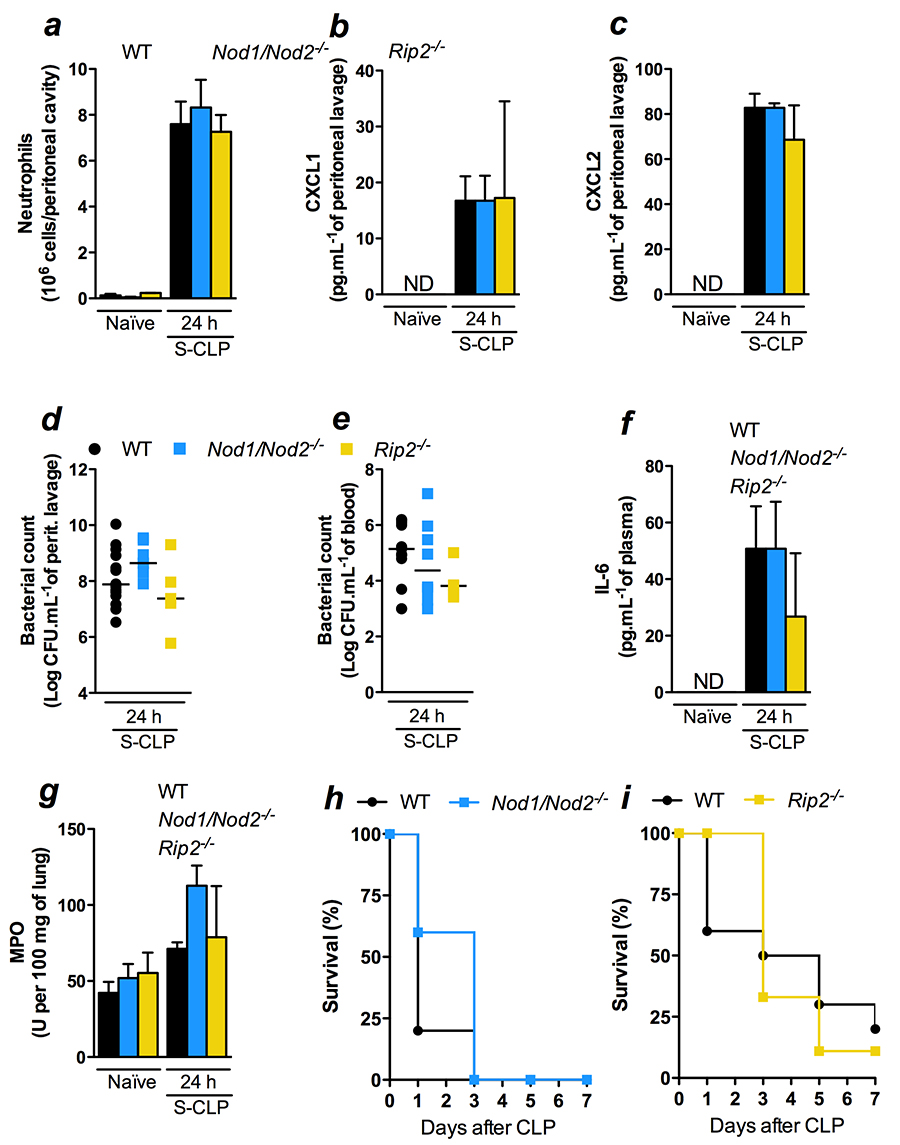
Figure
2:The additive response of
Nod1 and Nod2 is not essential to the inflammatory response during
severe polymicrobial sepsis. At 24h
Nod1/Nod2-and Rip2-deficient mice
(Nod1/Nod2−/− and
Rip2−/−,
respectively) underwent CLP they were assessed for: a) neutrophil
recruitment to the peritoneal cavity; b) CXCL1 and c) CXCL2 levels
in the peritoneal lavage as measured by ELISA; d) bacterial count
in peritoneal lavage and e) blood; f) IL-6 levels in plasma; g)
lung MPO activity; h) survival of WT and Nod1/Nod2-deficient mice and i) Rip2-deficient mice
post-CLP-induced severe sepsis. The data are expressed as the mean
± SEM in a, b, c, f and g; median in d and e; and as a percentage
of survival in h and i. The data in a, b, c, d, e, f and g were
analysed by multifactorial ANOVA and the data in h and i were
analysed by Mantel-Cox log-rank test. The results are
representative of at least two independent experiments. n = 5 to 8;
ND = not detected.
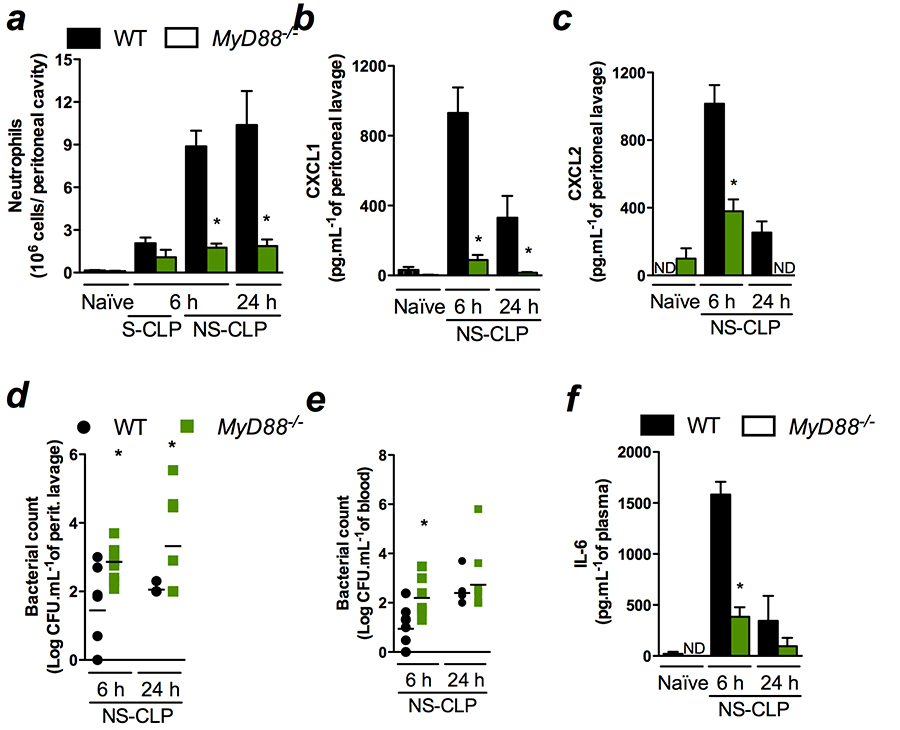
Figure
3:MyD88 is crucial for the
resolution of non-severe sepsis. Non-severe
(NS) and severe (S) sepsis were induced by CLP in WT and
MyD88-deficient mice and 6 or 24 h after sepsis induction, the
following were assessed: a) neutrophil recruitment to the
peritoneal cavity; b) CXCL1 and c) CXCL2 levels in the peritoneal
lavage determined by ELISA; d) bacterial count in the peritoneal
lavage and e) blood; f) IL-6 levels in the plasma, as measured by
ELISA. These data are expressed as the mean ± SEM in a, b, c, and f
and median in d and e. The data were analysed by multifactorial
ANOVA followed by unpaired t test. The graphs are representative of
one to four independent experiments. n = 4 to 10; ND = not
detected.*P<0.05.
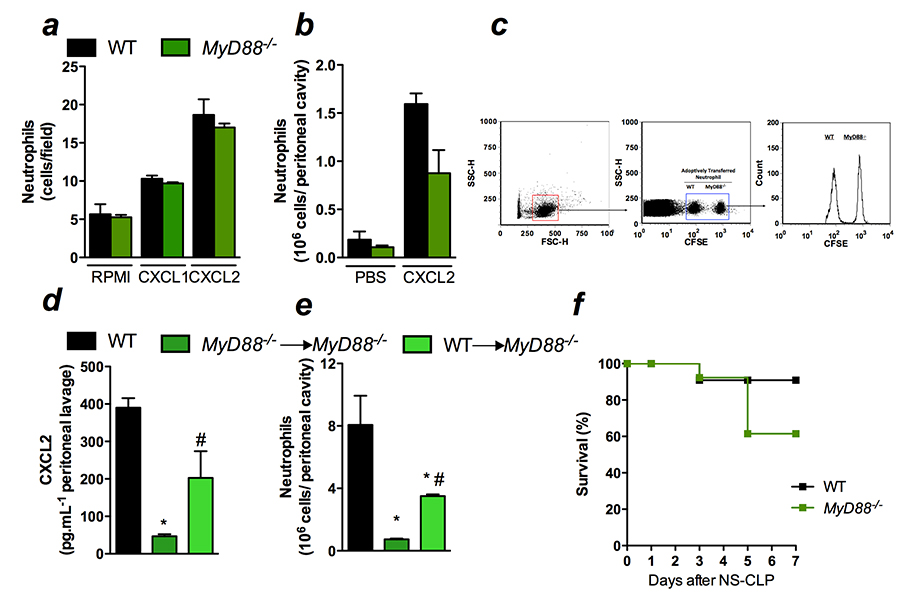
Figure
4:MyD88 is crucial for the
establishment of the inflammatory response during polymicrobial
sepsis. a) Bone
marrow-isolated neutrophils (5×104/well) from WT
or MyD88-deficient mice (MyD88−/−) were
stimulated by CXCL1 or CXCL2 (10 ng/mL) in a Boyden chamber to
measure chemotaxis. b) Neutrophil recruitment to the peritoneal
cavity 6 h after an i.p. injection of CXCL2 (30 ng/cavity). c)
Neutrophils from the bone marrow of WT or MyD88-deficient mice were
stained with different concentrations of CFSE and administered
(5×106/mouse; i.v.)
into WT mice 2 h before non-severe (NS) sepsis induction by CLP.
Cells in the peritoneal lavage were harvested 6 h after CLP surgery
and analysed by flow cytometry. For d–e, resident peritoneal cells
from WT and MyD88-deficient mice
were harvested and transferred (5×106/intraperitoneal cavity) to MyD88-deficient mice
30 minutes before CLP surgery. d) CXCL2 was measured by ELISA in
the peritoneal lavage at 6 h after CLP surgery, as was the e)
neutrophil recruitment to the peritoneal cavity. The data are
expressed as the mean ± SEM and were analysed by unpaired t test.
f) Sepsis was induced in WT
and MyD88-deficient mice (WT, MyD88−/−,
respectively) using CLP model. The survival curve was observed up
to 10 days after the induction of non-severe (NS) sepsis. The
results are expressed as percentage of survival and were analysed
by Mantel-Cox log-rank test. The graphs represent one of one to two
independent experiments. n = 5 to 6 per
experiment. *P<0.05 compared to WT and #P<0.05
compared to MyD88−/−→MyD88−/−.
COMMENTS FROM
AUTHOR
The recognition of
bacteria during an infection is crucial for the establishment of an
immune response. During this process, neutrophils are recruited to
the infection site to control bacterial growth. The impairment of
the neutrophil recruitment has been strongly associated to a poor
outcome to sepsis. The main families involved in the recognition of
pathogens are Toll-like receptors (TLR) and Nod-like receptors
(NLR). Nod1 and Nod2 have been previously shown to be involved in
neutrophil recruitment during bacterial infections. However, here
we clearly demonstrated that the absence of Nod1 and Nod2 are not
indispensable for the establishment of a local inflammatory
response, and neutrophil recruitment in sepsis. By contrast, we
showed that MyD88-dependent TLR signalling plays a crucial role in
the local production of inflammatory mediators and the consequent
recruitment of neutrophils to the infection site, preventing
mortality. Therefore, this study contributes to the further
understanding of the sepsis physiopathology, by describing the
differing involvement of the two main families of pattern
recognition receptors during the establishment of the immune
response.
LOCAL ADMINISTRATION OF GOLD NANOPARTICLES PREVENTS PIVOTAL PATHOLOGICAL CHANGES IN MURINE MODELS OF ATOPIC ASTHMA
Journal of Biomedical Nanotechnology11 (2015)1038-1050. [doi.org/10.1166/jbn.2015.2024]
Emiliano Barreto;Magda Fraguas Serra; Rafael Vitaldos Santos; Cássio Eráclito Alvesdos Santos;Jandir Hickmann;Amanda Costa Cotias; Camila Ribeiro Rodrigues Pão; Suelen GaunaTrindade; Vanessa Schimidt; Cristiano Giacomelli; Vinicius Frias Carvalho; Patricia M. R. Silva; Renato Sérgio Balão Cordeiro; Marco Aurélio Martins.
Atopic asthma is a chronic inflammation of the lung airways triggered by environmental antigens in genetically predisposed individuals. The prevalence of asthma has increased over the last 50 years, impacting around 300 million individuals worldwide and causing 250,000 annual deaths globally. Asthma pathogenesis is accounted for by a complex interplay of numerous cell types and inflammatory mediators, leading to airway hyper-reactivity (AHR), lung tissue remodeling and airflow limitation. T cells and T-helper 2 (Th2) cytokines orchestrate this complex response in which infiltrating eosinophils, and in some occasions also neutrophils, are suggested to play a major effector role.
Nanoscale structures can exhibit widely different properties to bulk materials or small molecules, which renders them applicable in the fields of medical imaging and therapy.On the other hand, it is well established that gold compounds have a variety of biomedical applications due to its anti-inflammatory and anti-oxidant activity.Remarkably, administration of gold nanoparticles can lead to anti-inflammatory effects in different pathophysiological conditions. For instance, they cause inhibition of inflammatory cell accumulation and reduction in TNF-α and IL-1β generation in experimental arthritis.Also, they are known to down-regulate the TLR4–NF-kB pathway by reducing oxidative damage in experimental uveitis triggered by LPS.Moreover, gold nanoparticles possess anti-angiogenic properties, as attested by impairment of both VEGF-induced migration of vascular endothelial cells in vitro and angiogenesis in nude mouse ear and mouse ovarian tumor experiments in vivo.
In the current study, we have employed outbred and inbred mouse strains, namely Swiss-Webster and A/J mice, respectively, to investigate the effect of intranasal instillation of low amounts of gold nanoparticles on allergen-induced lung inflammation, mucus exacerbation, subepithelial fibrosis and airway hyper-reactivity.
We have used small angle X-ray scattering (SAXS) and zeta-potential measurements in order to determine shape, size, dispersity and average zeta-potential of citrate-stabilized gold nanoparticles used in this study. As shown in Figure 1, their characteristic SAXS intensity profile was typical of scattering objects consisting of widely separated homogenous hard-spheres.
Ovalbumin provocation of sensitized A/J mice caused significant airway hyper-reactivity, as demonstrated by increased lung resistance and elastance after methacholine provocation (Figure 2). As shown in the same figure, intranasal treatments with gold nanoparticles prevented allergen-induced airway hyper-reactivity.
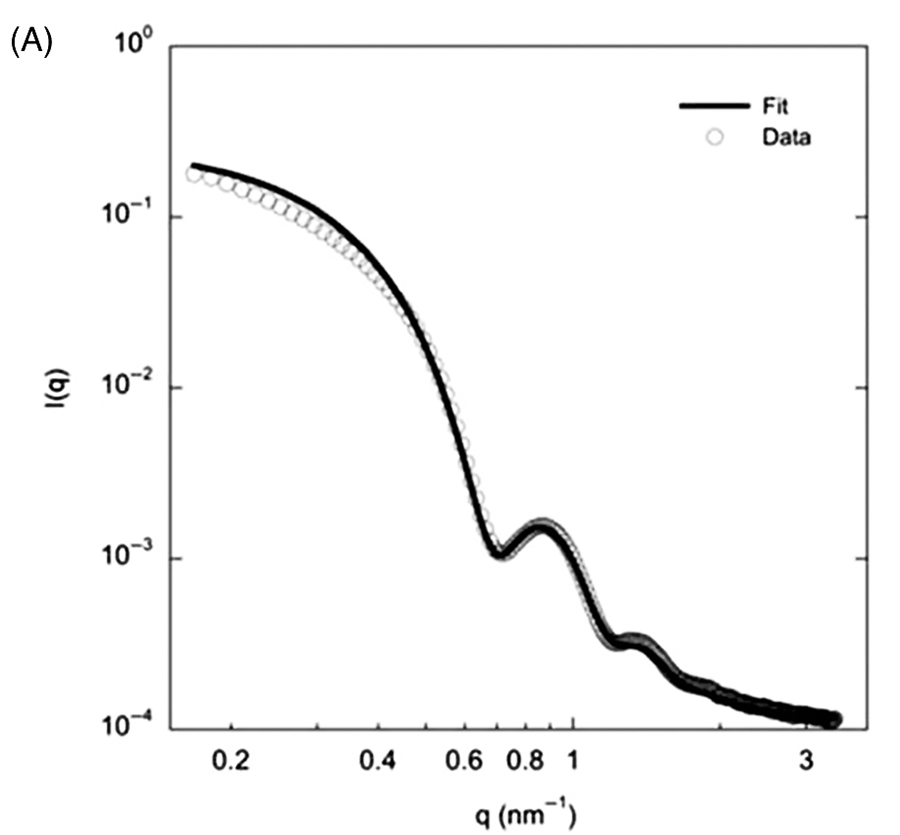
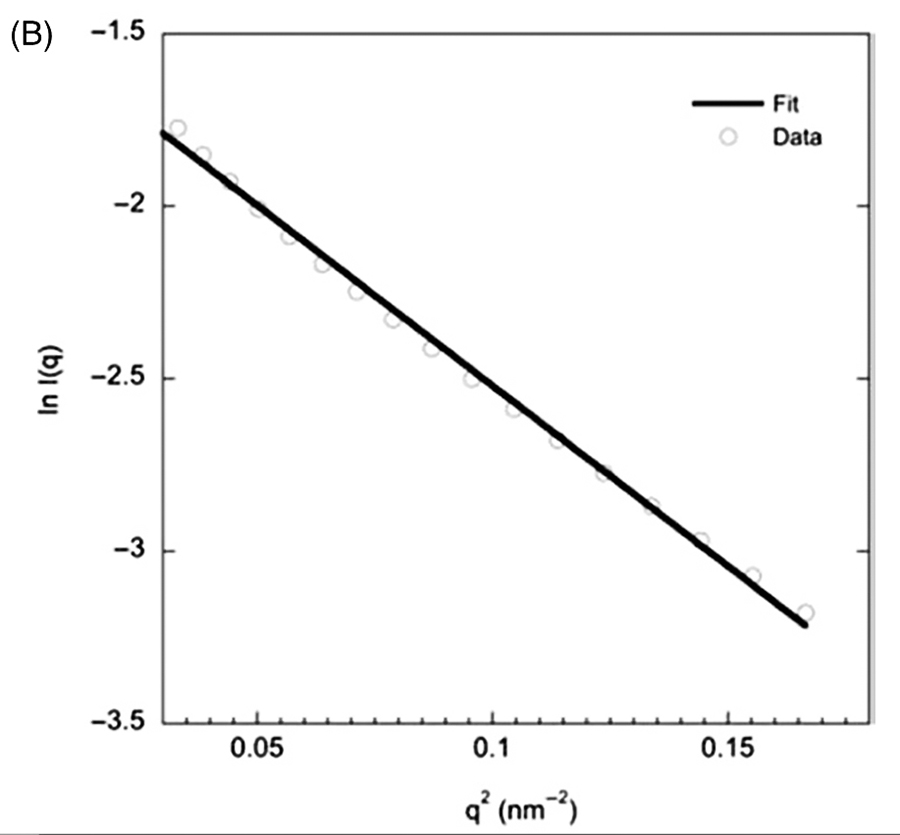
Figure 1. SAXS scattering profile with corresponding curve fitting (A) and Guinier plot (B) for 0.05 mg/mL AuNPs stabilized by 1.0 mM citrate species. Representative TEM micrographs of AuNP (C).
Next, we examined the anti-inflammatory and anti-remodelling effects of the nanoparticles. Lung tissue was collected 24 h after the last ovalbumin challenge in sen- sitized and challenged A/J mice. In ovalbumin-induced asthmatic mice, we observed an intense eosinophilic (cells stained in orange) leukocyte infiltration into the perivas- cular and peribronchiolar areas (Fig. 3B), as compared to the sham-challenged group (Fig. 3A). This inflammatory response was significantly inhibited in animals pretreated with gold nanoparticles (60 μg/kg) (Fig. 3C)), reaching a blockade of about 55% 24 h post-challenge (Fig. 3D).
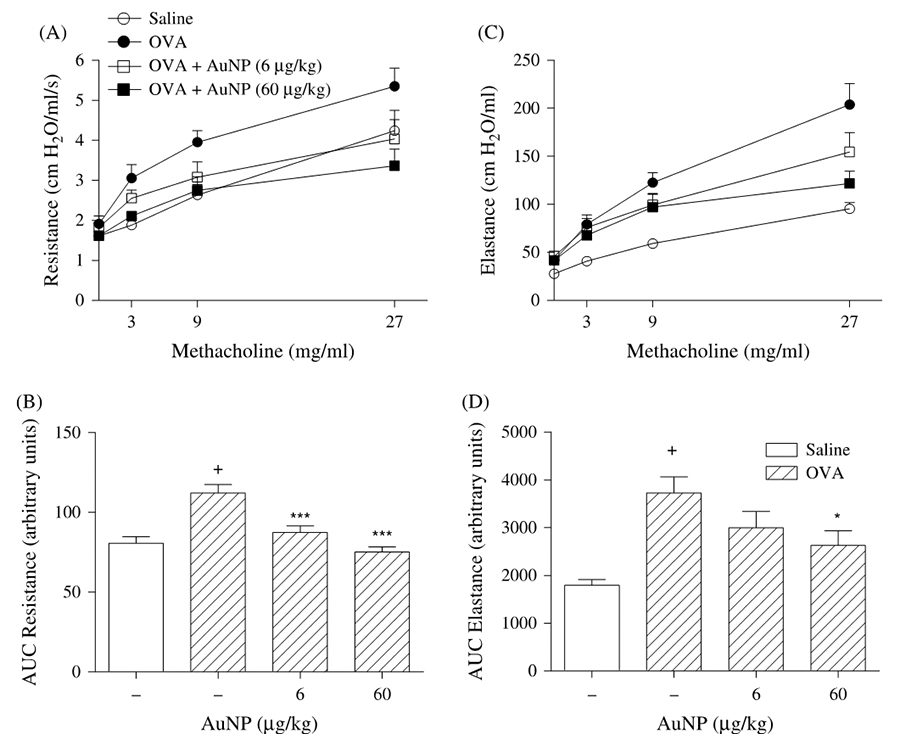
Figure 2. AuNP attenuates the ovalbumin-induced AHR caused by methacholine aerosolization of sensitized and challenged A/J mice. AHR was monitorized by assessing changes in airway resistance ((A), (B)) and elastance ((C), (D)) induced by increasing concentrations of methacholine 24h after the last ovalbumin or saline challenge. (B) and (D) represent area under the curve calculated from the dose-response curves of bronchoconstriction to methacholine. Data are expressed as mean ± S.E.M. of mice. +P < 0.001 as compared to the sham-challenged group. *P < 0.05 and ***P < 0.001 as compared to the ovalbumin-challenged group.
Our data further revealed that gold nanoparticles significantly reduces the mucus hyper-secretion(Fig. 4), as well adverse airway remodelling (Fig. 5), pointed out by over-deposition of extracellular matrix in the lung sub-epithelial area of allergen-challenged animals. In addition, the protective effects of gold nanoparticles administered locally correlated with the blockade of a range of Th2 pro-inflammatory cytokines and chemokines, also confirmed in an outbred strain of mice, named Swiss-Webster (data not shown).
Asthma pathological changes are associated with airway inflammation and oxidative stress in many ways. Remarkably, the treatment led to significant reduction in the generation of free radicals, including ROS and MDA. Allergen provocation led to a significant increase in the levels of lung tissue lipid peroxidation, which was quantified by MDA formation. MDA levels in these samples increased from 71.4 ± 2.4 (n = 6) to 100.5 ± 3.8 nmol/mg of protein (mean ± S.E.M.) (N = 6) (p< 0.001) in sham- and allergen-challenged mice, respectively. Upon gold nanoparticle treatment (60 μg/kg), a significant reduction in the levels of MDA was observed in the lung of allergen-challenged mice (84.8 ± 1.7 nmol/mg of protein; mean ± S.E.M.) (N = 6) (p< 0.01).
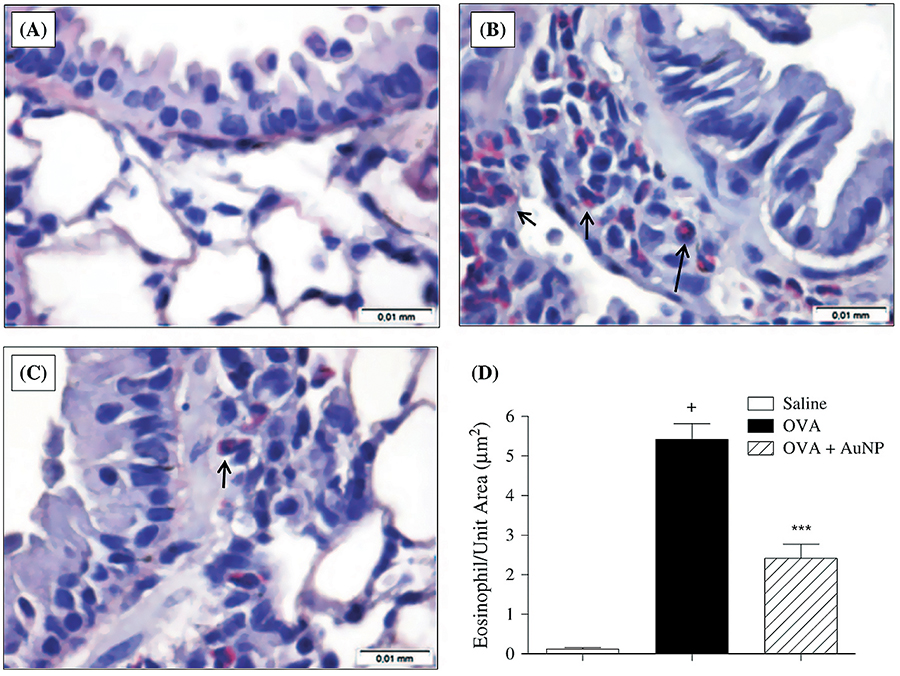
Figure 3. Effect of AuNPs on lung tissue inflammatory cell infiltration in A/J mice. Panels show photomicrographs of lung preparations stained with Llewellyn’s Sirus Red from the sham-challenged (A), ovalbumin-challenged (B) and AuNP-treated (60 μg/kg) ovalbumin-challenged mice (C). Eosinophil numbers are shown in panel (D). Data are expressed as mean ± S.E.M. of 6 mice. +P < 0.001 as compared to the sham-challenged group. ***P < 0.001 as compared to the ovalbumin-challenged group.
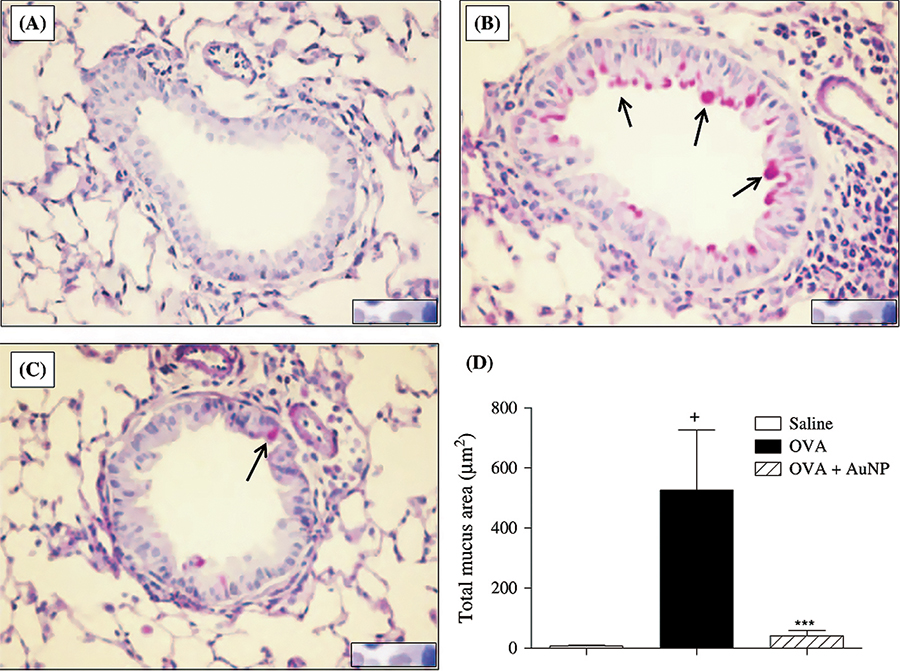
Figure 4. AuNPs reduced mucus production in allergen-challenged A/J mice. Panels show photomicrographs of lung preparations stained with Periodic Acid-Schiff (PAS), from sham-challenged (A), ovalbumin-challenged (B) and AuNP-treated (60 μg/kg) ovalbumin-challenged mice (C). Quantitative mucus production is seen in panel (D). Data are expressed as mean ± S.E.M. of 6 mice. +P < 0.001 as compared to the sham-challenged group. ***P < 0.001 as compared to allergen-challenged group.
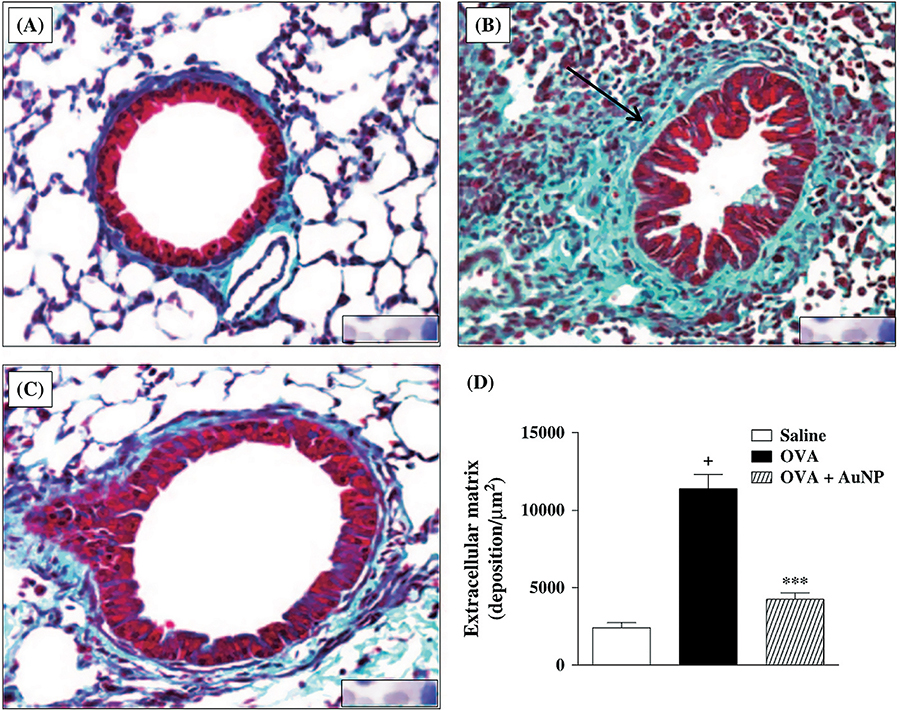
Figure 5. AuNP decreases airway extracellular matrix deposition triggered by allergen in A/J mice. Panels show photomicro-graphs of lung preparations stained qith Gomori trichrome from the saline-challenged (A), ovalbumin-challenged (B) and AuNP-treated (60 μg/kg) ovalbumin-challenged mice (C). Quantitative extracellular matrix deposition is panel (D). Data are expressed as mean ± S.E.M. of mice. +P < 0.001 as compared to the sham-challenged group. ***P < 0.001as compared to the ovalbumin-challenged group.
Altogether, these results are in line with the interpretation that gold nanoparticles have indeed clinical potential as anti-asthma therapy. A possible antioxidant effect of gold nanoparticles may protect the lung tissue against the injurious oxidants agents induced after allergen challenge, and also may alter the inflammatory events, which have a central role in the pathogenesis of airway and lung diseases.
COMMENTS FROM
AUTHOR
Inflammation is pivotal in lung chronic diseases, such as asthma, which have high socioeconomic impact worldwide and in Brazil, and can be fatal. Inhaled glucocorticoids are the most effective treatment to control asthma so far, but adverse effects and resistance to anti-inflammatory steroids limit their effectiveness.The use of metallic gold or its complexes for the treatment of different inflammatory conditions has several thousand years of history. Nanotechnology provides novel materials in the nanometer range with putative applications in clinical settings. Using distinct animal models of asthma, we provided evidence that gold nanoparticles have indeed marked antiasthma properties, clearly associated with their anti-inflammatory and antioxidant activities. Findings that intranasal administration of gold nanoparticles can robustly inhibit several pathological features oft his disease, including pulmonary inflammation, airway hyper-reactivity, mucus exacerbation and lung remodeling are suggestive that this treatment can be beneficial for asthmatics. In addition, the unique properties of biocompatibility, high surface of reactivity and flexibility in functionalization may further support novel clinical applications for this millenary and precious material.
ANTI-INFLAMMATORY PROPERTIES OF
CONVOLUTAMYDINE A AND TWO STRUCTURAL ANALOGUES
Life Sciences 116
(2014) 16–24. http://dx.doi.org/10.1016/j.lfs.2014.08.019
Patricia D.
Fernandes,
Renata S.
Zardo,
Gabriella S.M.
Figueiredo,
Bárbara V.
Silva,
Angelo C.
Pintob
Inflammation is
a stereotyped response of living organisms to a possible harmful
stimuliin vascular
tissues. This physiologic event has a
protective role aiming at the removal of the offending agent by
activating a cascade of events and production of inflammatory
mediators. The ultimate goal is the destruction of the offending agent and
tissue restoration. The
current pharmacological therapies for inflammation consist
primarily of non-steroidal anti-inflammatory drugs (NSAIDs). This
class has a long history of clinical use and major deficiencies,
and many of the drug discovery efforts in the area of inflammation
have focused on the incremental improvement of this class of
compounds.
However, adverse side-effects (i.e., ulcers and
bleeding) have limited their use. Many new
molecules have been synthesized with the aim of seeking those with
reduced side effects.
Amathia
convolutais a
marine bryozoan species from which
the oxindole alkaloid named Convolutamydine A was isolated. Because
convolutamydine is only isolated from this Bryozoan in small
amountsanddue to
its promising biological
activities, it has been synthesized by several
research groups.
Garden et al. were the first group
to propose the synthesis of a
racemic mixture of convolutamydine A from isatin, a small, versatile, and widely applicable
pharmacological molecule.
Convolutamydine
A promoted alterations in HL-60 cell line..In
addition to convolutamydine A, the literature includes reports of
other 3-substituted-3-hydroxyindolin-2-ones with applications in
medicinal chemistry, such as active potent growth hormone secretagogues, potassium
channel openers, anticonvulsants and antinociceptives.
In our continuous search for
bioactive substances, the purpose of the present work was to
investigate the anti-inflammatory effects of convolutamydine A and
its analogues in animal models of inflammation.
The pretreatment of mice with
0.01, 0.1 or 1 mg/kg of each compound demonstrated that
convolutamydine A and ISA003 significantly reduced the licking
response to formalin injection at all doses tested, whereas ISA147
did not affect the response (Figure 1).
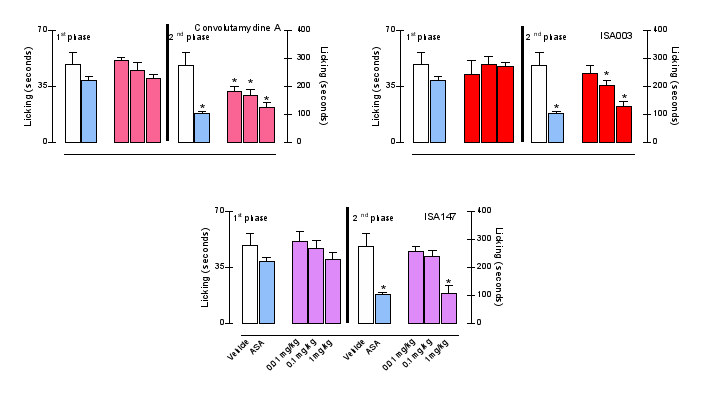
Figure
1: The effects of
Convolutamydine A, ISA003 and ISA147
on the formalin-induced licking response in mice. Convolutamydine A
or analogues 0.01, 0.1 or 1 mg/kg),acetylsalicylic acid (ASA, 100 mg/kg) or vehicle were
orally administered to mice. Values mean ± S.D. (n=6–10).
*P<0.05 compared to vehicle-treated mice using ANOVA followed by
Bonferroni's test.
The inhibitory
effect observed for convolutamydine A and its analogues is
indicative of a possible anti-inflammatory effect. Accordingly, we
evaluated convolutamydine A, ISA003 and ISA147 in another model of
inflammation, the subcutaneous air pouch (SAP) model.
The injection of carrageenan (1%)
into the SAP produced a marked increase in the exudate volume and
leukocyte number in the pouch, accompanied by an increase in almost
seven-fold above the level of the control group (PBS injected into
the SAP). The pre-treatment of mice with convolutamydine A or its
analogues (at doses of 0.1, 1 or 10 mg/kg) significantly
suppressed the number of leukocytes in the exudates.
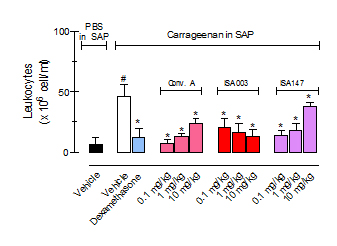
Figure 2: Effects of convolutamydine A (Conv. A), ISA003 and ISA147 in
leukocyte migration into the
subcutaneous air pouch (SAP). The animals were pretreated with
different doses of each substance, dexamethasone (5 mg/kg, i.p.) or
vehicle 1 h before carrageenan (1%) injection into the SAP. Values
mean ± S.D. (n=6–10). #P<0.05
compared to vehicle-treated mice receiving PBS in SAP and
*p<0.05 compared to vehicle-treated mice receiving carrageenan
in SAP using ANOVA followed by Bonferroni's test.
Because
convolutamydine A and its analogues significantly reduced cell
migration into the SAP, we decided to further analyse other
parameters that are present in carrageenan-induced inflammation. In
this regard, we measured the amount of some cytokines (i.e., TNF-α
and IL-6) in the exudates. Carrageenan induced 2.6- and 0.7-fold
increases in the amount of TNF-α and IL-6, respectively.
All tested doses of convolutamydine A
and ISA147 (0.1, 1 and 10 mg/kg) reduced cytokine levels, whereas
ISA003 only showed an effect at the 1 or 10 mg/kg doses (Figure
3).
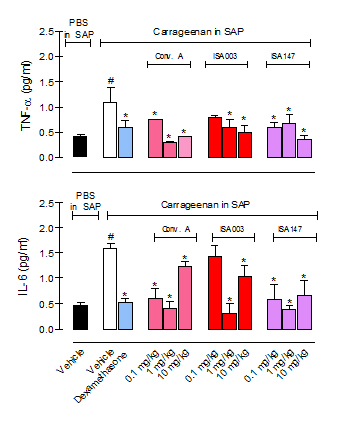
Figure
3: Effect of convolutamydine A (Conv. A), ISA003 and ISA147on
carrageenan-induced TNF-α, IL-6 and
nitric oxide (NO) production in the subcutaneous air pouch (SAP).
The animals were pretreated with different doses of each substance,
dexamethasone (5 mg/kg, i.p.) or vehicle. The results are presented
as the mean ± S.D. (n= 6-10). #P<0.05
compared to vehicle-treated mice receiving PBS in SAP and
*p<0.05 compared to vehicle-treated mice receiving carrageenan
in SAP using ANOVA followed by Bonferroni's test.
Figure 4 shows that both
convolutamydine A and its analogues significantly and
dose-dependently reduced NO and PGE2 production by the leukocytes
that migrated into the SAP after carrageenan injection.
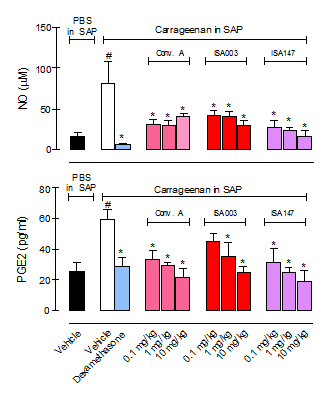
Figure
4: The effect
of convolutamydine A (Conv. A), ISA003 and ISA147on
levels of nitric oxide (NO) and
prostaglandin E2 (PGE2) accumulated in the subcutaneous air pouch
(SAP). The animals were pretreated with different doses of each
substance, dexamethasone (5 mg/kg, i.p.) or vehicle. The results
are presented as the mean ± S.D. (n= 6-10).
#P<0.05 compared to vehicle-treated mice receiving
PBS in SAP and *p<0.05 compared to vehicle-treated mice
receiving carrageenan in SAP using ANOVA followed by Bonferroni's
test.
To further evaluate the effect of
convolutamydine A and its analogues and to eliminate the
possibility that the reduction in cytokines, NO and
PGE2in vivo
could simply be the result of a
reduction in the number of cells that migrated into the SAP, we
decided to carry out in
vitro assays. We first
investigated a possible direct cytotoxic effect of convolutamydine
A, ISA003 and ISA147on RAW 264.7 cells. Neither the compounds
alone, at concentrations up to 100 μM, nor the compounds in the
presence of LPS (1 μg/ml) affected cell viability (data not shown).
To examine if the inhibitory effects of convolutamydine A, ISA003
and ISA147 occurred due to an inhibition of inducible nitric oxide
synthase (iNOS) and cyclooxygenase-2 (COX2) expression, the levels
of each protein was determined by western blot analysis. As shown
in Figure 7, the expression of the iNOS and COX-2 proteins was
almost undetectable in unstimulated cells. However, upon LPS
treatment, the iNOS and COX-2 proteins were markedly increased.
Convolutamydine A, ISA003 and ISA147 significantly reduced the
induction of iNOS and COX-2 protein expression (Figure
5).
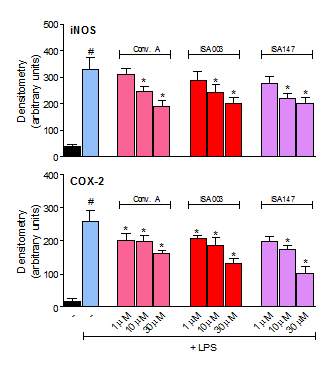
Figure 5
: The effects of
convolutamydine A, ISA003 and ISA147 on iNOS or cyclooxygenase-2
(COX-2) enzyme expression in RAW 264.7 cells. RAW 264.7 cells were
activated or not with LPS (1 μg/ml) and then incubated with the
compounds (1, 10 or 30 μM). After 6 h of incubation, western blot
analysis was performed to visualise iNOS and COX-2 levels. The
results are presented as the mean ± S.D. (n=4). Statistical
significance was calculated by ANOVA followed by Bonferroni's
test. #P<0.05 when
comparing LPS-activated with non-activated cells; *P<0.05 when
comparing the LPS-activated cells that were pre-incubated with
compounds to the LPS-activated cells.
COMMENTS FROM
AUTHOR
Two new analogs from
Convolutamydine A, named ISA003 and ISA147, were evaluated in model
of acute inflammation.The
anti-inflammatory effects of convolutamydine A, ISA003 and ISA147
were investigated in a formalin-induced licking behavior model,
where mice received an intraplantar injection of formalin and their
licking behavior was evaluated for 30 min. Additionally,
inflammatory parameters were evaluated in a subcutaneous air pouch
(SAP) model of carrageenan-induced inflammation. Exudates were
collected for leukocyte counts; measurement of protein,
prostaglandin E2 (PGE2) and cytokines by ELISA; and analysis of
nitric oxide (NO) using a nitrate conversion
protocol. Cyclooxygenase-2 (COX2) and inducible nitric oxide
synthase (iNOS) from RAW 264.7 cells were quantified by
immunoblotting. Convolutamydine A and its two analogues inhibited
the formalin-induced licking response at doses as low as 0.01
mg/kg. An inhibitory effect was also observed on leukocyte
migration and the production of NO, PGE2 and cytokines (IL-6 and
TNF-α). The reduction in inflammatory parameters did not appear to
be correlated with a direct reduction in the number of cells in the
SAP, because a reduction in NO and PGE2 production by cultured
macrophages was observed in addition to the inhibition of iNOS and
COX2 enzyme expression. These results indicate that convolutamydine
A and its two analogues have significant anti-inflammatory effects.
These substances can be improved to generate lead compounds for the
synthesis of new anti-inflammatory drugs.
DONEPEZIL: AN IMPORTANT PROTOTYPE TO THE
DESIGN OF NEW DRUG CANDIDATES FOR ALZHEIMER’S DISEASE
Mini-Reviews in Medicinal
Chemistry, 2014,
14, 2-19 [DOI: 10.2174/1389557513666131119201353]
Maria Cecília Rodrigues Simões,
Flávia Pereira Dias Viegas, Marcella Soares Moreira, Matheus de
Freitas Silva, Mariana Máximo Riquiel, Patrícia Mattos da Rosa,
Maísa Rosa Castelli, Marcelo Henrique dos Santos, Marisi Gomes
Soares and Claudio Viegas Jr.
Alzheimer’s Disease (AD) is
a neurodegenerative disorder characterized by an insidious onset
and a complex chronic multi-factorial progress, affecting
hippocampus and frontal cortex in brain. This devastating pathology
manifests its symptoms as a severe loss in memory, language skills
decline and other cognitive impairments, with dramatic behavioral
changes that progress to depression and, eventually, death. Recent
data points that AD is responsible for ca. 50-60% of all cases of
dementia in people over age 65. It is estimated that more than 4.5
million people have AD in the US and 18 million worldwide. The
etiology of AD remains unclear, however many pathophysiologic
hallmarks of the disease have been disclosed and are currently well
established.
They involve a complex network of interconnected
factors such as a rapid onset of cholinergic dysfunction, with
remarkable depletion of acetylcholine (ACh), accomplished by
aggregation and accumulation of extracellular β-amyloid (βA)
peptide as senile neuritic plaques, and intracellular formation of
neurofibrillary tangles (NFTs), composed by a hyperphosphorylated
form of the microtubule-associated protein tau, oxidative stress,
and neuronal loss. The Ca2+ion plays an important role in
the cerebral homeostasis, acting as a second messenger in the
brain. The imbalance of Ca2+is currently considered one of
the main causes of neurodegeneration due to A_ effects on the
capacity of membrane cells to regulate their permeability and
internal concentration of ions Ca2+. The
Ca2+-associated neurodegeneration begins when Aβ causes an
increase in the ion influx as a result of the activation of
the N-Methyl-Daspartate receptors by the neurotransmitter
glutamate. Besides cognitive and motor changes, AD patients also
present diverse behavioral alterations as irritability, anxiety,
depression, disorientation and restlessness. To date, AD remains
incurable and with few available therapeutic alternatives to
ameliorate cognition and life quality of the patient, arousing
special attention and efforts in the search for new effective
drugs. All current drugs available for the treatment of AD are only
symptomatic, acting mainly as acetylcholinesterase inhibitors
(AChEIs) [3b]. Drugs from this therapeutic class are supported by
the “Cholinergic Hypothesis” that points to restore the cholinergic
deficit in central nervous system (CNS) by selective inhibition of
AChE enzyme, and thus result in a delay of the cognitive decline
and in the control of AD symptoms. During the last two decades,
only few anticholinergic drugs have been launched in the market,
and are mainly indicated for the treatment of mild and moderate
stages of the disease, such as tacrine, donepezil, rivastigmine and
galanthamine. Another drug recently approved by FDA is memantine,
that acts as na antagonist of glutamate receptors, being indicated
for the treatment of moderate and severe stages of AD.
However, due to a number of
adverse peripheral effects arising from the excessive activation of
cholinergic system, including confusion, hallucinations, behavioral
abnormalities, nausea, gastric irritation and hepatotoxicity, these
drugs have a quite limited clinical use. Besides the treatment with
AChEIs and memantine, many other therapeutic approaches, such as
the use of neurotrophic and anti-inflammatory drugs, antioxidant
compounds and formulations, compounds that could interfere in
Aβ-aggregation process have been exploited in the search for new
effective therapeutic alternatives. In this context, the more
recent approach that has emerged to support the design of more
effective chemical entities have considered the multifactorial and
complex interconnected and, in some cases parallel or simultaneous,
biochemical pathways in AD. This strategy is called Multi-target
directed ligands (MTDLs) or multifunctional ligands, that is based
on the fact that using a one-target-direct drug, it is not Always
likely that the therapeutical effect will be effective to block
disease evolution. In the structure of
donepezil, N-benzylpiperidine and indanone moieties were identified as
important interaction binding sites with AChE, and are responsible
for inhibitory selectivity. In spite of donepezil had been
developed as a racemic mixture, with both enantiomers exhibiting
the same activity, the eutomer is the R isomer, which exhibited 5-fold
more affinity for AChE (Ki = 3.35 nM) than the S isomer (Ki = 17.5 nM)
[10]. Donepezil is recognized by
AChE by interactions in the middle gorge of the active site of the
enzyme, mainly by three subunits: the benzyl moiety, the nitrogen
atom at the piperidine ring, and the dimethoxyindanone portion.
These interactions involve direct contacts mediated by water
molecules that seem to be crucial for binding and
specificity. During the last two decades,
Alzheimer’s disease has been the focus of enormous scientific and
medical efforts and investments around the world, in a run against
the time for more effective and secure medicines and, the so
waited, discovery for cure. This neuropathology has great social
and economic impacts, due to the disastrous functional and
behavioral impairments caused in patients, that usually have death
as the end point, after 8-10 years after the first symptoms of AD
are recognized. Since donepezil has been launched in the Market in
1996s, this drug has attracted special attention due to its AChE
inhibitory potency, high selectivity, low toxicity and good
bioavailability. Thus, donepezil has also been exploited as
molecular scaffold for design and development of new AChE inhibitor
drug candidates. Moreover, molecular hybridization has been the
main approach for rational drug design of new ligands, capable to
act as dual, multipotent and/or multi-target directed mechanisms of
action.
COMMENTS FROM
AUTHOR
After discovery of Donepezil and its approval
for the treatment of Alzheimer disease, this drug has gained
special importance due to its low side effects and much better
effectiveness against AD. For this reason, structural features of
donepezil have been exploited in the search of new anti-Alzheimer
drug candidates trying to reproduce its AChE inhibitory properties
and also introducing other structural attributes to ensure a
multiple targets profile of action. This approach have been used in
our group in the last decade, aiming to produce novel drug
candidates prototypes planned by molecular hybridization with other
antioxidant, anti-inflammatory, metal scavengers and
neuroprotective natural and synthetic compounds. This strategy
allowed us the discovery of few active molecules, with multi-target
directed profiles that are under pre-clinical investigation for
their effectiveness in AD treatment. In this context, we are
searching for novel molecules with new mechanisms of action that
could represent radical innovation on AD therapy.
INCT-INOFAR PUBLICATIONS
Scientific Articles
Brazilian Publications
National Journals
1. Oliveira CA, Souza ACJ, Santos APB, Silva BV, Lachter ER, Pinto AC. Synthesis of Fruity Flavor Esters: An Experiment for Undergraduate Courses within one of the Principles of Green Chemistry. Revista Virtual de Química, v. 6, p. 152-167, 2014.[LINK]
2. Paumgartten FJR. Thalidomide and its analogues: comparative clinical efficacy and safety, and cost-effectiveness. Cadernos de Saúde Pública (ENSP. Impresso), v. 30, p. 684-686, 2014. [DOI]
3. França RRF, De Carvalho AS, Branco FSC, Pinto AC, Boechat N. Potent Inhibitors of the Enzyme Sterol 14α-demethylase AgainstT. cruzi.Revista Virtual de Química, v. 6, p. 1483-1516, 2014.
4. Pinto AC. Science City (Editorial).Revista Virtual de Química. v.6, p.168-168, 2014.
5. Pinto AC. Indicadores X qualidade: O dilema atual dos cientistas. Revista Virtual de Química. v. 6, p.833-833, 2014.
6. Britto ALS,Antunes AMS. A Regulação da Industria de Petróleo, gás Natural e Biocombustíveis e a sua contribuição para o sistema setorial de inovação. Cadernos de Prospecção, v. 7, p. 324-334, 2014.
7. Magalhães JL, Cartaxo RJA,Antunes AMS. Produção de Fármacos & Medicamentos no Brasil: uma proposta de metodologia para priorização da Lista Estratégica no Âmbito do SUS. RECIIS. Revista Eletrônica de Comunicação, Informação & Inovação em Saúde (Edição em Português. Online), v.8, p. 478-495, 2014.
8. Delgado IF, Paumgartten FJR. Effects of Euphorbia milii latex on mitogen-induced lymphocyte proliferation.Revista Brasileira de Plantas Medicinais (Impresso), v. 16, p. 107-111, 2014.[DOI]
9. Paumgartten FJR,Moreira DL. Illicit drugs, legal highs and over-consumption of psychoactive medicines: the many faces of a public health problem. Cadernos de Saúde Pública (ENSP. Impresso), v. 30, p. 1349-1350, 2014.[DOI]
10. Moreira DL, Teixeira SS, Monteiro MHD, de Oliveira ACAX, Paumgartten FJR. Traditional use andsafetyofherbal medicines. Revista Brasileira de Farmacognosia (Impresso), v. 24, p. 248-257, 2014.[DOI]
11. Coelho DR, Miranda ES, Saint’Pierre TD, Paumgartten FJR. Tissue distribution of residual antimony in rats treated with multiple doses of meglumineantimoniate. Memórias do Instituto Oswaldo Cruz (Impresso), v. 109, p. 420-427, 2014.[LINK]
12. Paumgartten FJR. Developmental risks associated with use of psychoactive drugs during pregnancy are largely unknown. Revista Brasileira de Psiquiatria (São Paulo. 1999. Impresso), v. 36, p. 359-360, 2014.[DOI]
13. Stolz ED, Müller LG, Trojan-Rodrigues M, Baumhardt E, Ritter MR, Rates SMK. Survey of plants popularly used for pain relief in Rio Grande doSul, southern Brazil. Revista Brasileira de Farmacognosia (Impresso), v. 24, p. 185-196, 2014.[DOI]
14. Foletto MC, Haas SE. Cutaneous melanoma: New advances in treatment. Anais Brasileiros de Dermatologia. v. 89, p. 301-310, 2014.[DOI]
15.Barros JIT, Fecgine FV, Montenegro Jr. RM, Vale OC, Fernandes VO, Souza MHLP, Cunha GH, Moraes MO, D’alva CB, Moraes MEA.Effect of treatment with sitagliptin on somatosensory-evoked potentials and metabolic control in patients with type 2 diabetes mellitus.Arquivos Brasileiros de Endocrinologia e Metabologia (Impresso), v. 58, p. 369-375, 2014.[DOI]
16. Dornelas CA, Cavalcanti BC, Magalhães HI, Jamacaru FV, Furtado FN, JuanesCde C, Melo Nde O, de Moraes MO. Potential chemoprotetive effects of green propólis, L-lysine and celecoxib on bone marrow cells and peripheral blood lymphocytes of Wistar rats subjected to bladder chemical carcinogenesis.Acta Cirúrgica Brasileira (Impresso), v. 29, p. 423-428, 2014.
17. Henriques MSM, Leite JA, Diniz MFFM, Araújo MST.Immunohistochemistry pathern of hepatic inflammatory and insulin resistance markers in experimental model of nonalcoholic steatohepatitis.Jornal Brasileiro de Patologia e Medicina Laboratorial (Impresso), v. 50, p. 136-143, 2014.[DOI]
Foreign Publications
International Journals
1. Pereira SL, Kummerle AE, Fraga CA, Barreiro EJ, Sudo RT, Zapata-Sudo G. N-Acylhydrazone improves exercise intolerance in rats submitted to myocardial infarction by the recovery of calcium homeostasis in skeletal muscle. Life Sciences (1973), v. 94, p. 30-36, 2014.[DOI]
2. Lacerda RB, Sales NM, da Siulva LL, Tesch R, Miranda ALP, Barreiro E J, Fernandes, PD, Fraga, CAM.Novel Potent Imidazo[1,2-a]pyridine-N-Glycinyl-Hydrazone Inhibitors of TNF-α Production: In Vitro and In Vivo Studies. PlosOne, v. 9, p. e91660, 2014.[DOI]
3. Lima CKF, Silva RM, Lacerda RB, Santos BLR, Silva RV, Amaral LS, Quintas LEM, Fraga CAM, Barreiro EJ, Guimaraes MZP, Miranda ALP.LASSBio-1135: A Dual TRPV1 Antagonist and Anti-TNF-Alpha Compound Orally Effective in Models of Inflammatory and Neuropathic Pain. PlosOne, v. 9, p. e99510, 2014. [DOI]
4. da Silva RB,da Silva EF, Carvalho AS, Fraga CA,Wardell SMSV,Wardell JL . Crystal structures of 1-hydroxylimidazole and imidazole 1-oxide derivatives. ZeitschriftfürKristallographie, v. 229, p. 709-722, 2014.[LINK]
5. Maia RC, Tesch R, Fraga CA.Acylhydrazonederivatives: a patentreview. Expert Opinion on Therapeutic Patents, v. 24, p. 1161-1170, 2014.[DOI]
6. Sato JAP,Costa FN,Da Rocha MD, Barreiro EJ,Fraga CA,PunzoF, Ferreira FF. Structural characterization of LASSBio-1289: A novel vasoactive N-methyl-N-acylhydrazone derivative. Cryst Eng Comm (Cambridge. Online), v. 17, p. 165-173, 2015. [DOI]
7. Carvalho AS,Kaiser M,Brun R,Silva EF, Fraga CA. Antiprotozoal Activityof (E)-Cinnamic N-Acylhydrazone Derivatives. Molecules (Basel. Online), v. 19, p. 20374-20381, 2014.[DOI]
8. Pereira SL, Kummerle AE, Fraga CA, Barreiro EJ, Sudo RT, Zapata-Sudo G. Vasodilator and antihypertensive effects of a novel N -acylhydrazone derivative mediated by the inhibition of L-type Ca 2+ channels. Fundamental & Clinical Pharmacology, v. 28, p. 29-41, 2014.[DOI]
9. Barbosa MLC, Lima LM,Tesch R, Sant'Anna CMR, Totzke F, Kubbutat MHG, Schachtele C, Laufer SA, Barreiro EJ.Novel 2-chloro-4-anilino-quinazoline derivatives as EGFR and VEGFR-2 dual inhibitors.European Journal of Medicinal Chemistry, v. 71, p. 1-14, 2014.[DOI]
10. Costa FN,Braz D,Ferreira FF, da Silva TF, Barreiro EJ,Lima LM, Colaço MV,Kuplich L,Barroso RC.Synchrotron X-ray powder diffraction data of LASSBio-1515: A new N-acylhydrazone derivative compound. Radiation Physicsand Chemistry (1993), v. 95, p. 292-295, 2014. [DOI]
11. Amaral DN,Cavalcanti BC,Bezerra DP,Ferreira PMP, Castro RP, Sabino JR, Machado CML, Chammas R,Pessoa C, Sant’anna CMR, Barreiro EJ, Lima LM. Docking, Synthesis and Antiproliferative Activity of N-Acylhydrazone Derivatives Designed as Combretastatin A4 Analogues. Plos One, v. 9, p. e85380, 2014.
12. Alencar AK, Pereira SL, da Silva FE, Mendes LV, Cunha Vdo M, Lima LM, Montagnoli TL, Caruso-Neves C, Ferraz EB, Tesch R, Nascimento JH, Sant'anna CMR, Fraga CA, Barreiro EJ, Sudo RT , Zapata-Sudo G.N-acylhydrazone derivative ameliorates monocrotaline-induced pulmonary hypertensionthrough the modulation of adenosine AA2R activity. International Journal of Cardiology (Print), v. 173, p. 154, 2014.[DOI]
13. da Silva YK, Reyes CT, Rivera G, Alves MA, Barreiro EJ, Alexandre-Moreira MS, Lima LM. 3-Aminothiophene-2-Acylhydrazones: Non-Toxic, Analgesic and Anti-Inflammatory Lead-Candidates. Molecules (Basel. Online), v. 19, p. 8456-8471, 2014.[DOI]
14. Nunes IKC,Tinoco LW, Martins HJ,Rezende C, Barreiro EJ,Lima LM. In Vitro Microsomal Hepatic Metabolism of Antiasthmatic Prototype LASSBio-448. Current Topics in Medicinal Chemistry (Print), v. 14, p. 1388-1398, 2014. [DOI]
15. Lima LM, Trachez MM, de Araújo JSC, da Silva JC, Amaral DN, Sudo RT, Barreiro EJ, Zapata-Sudo G.Novel PartialAgonistof PPAR-Gamma for Treatment of Diabetic Neuropathy in Rats. Journal of Diabetes & Metabolism, v. 05, p. 392-396, 2014. [DOI]
16. Araújo GL, Vieira AE, Barreiro EJ, Lima LM, Cardoso CN, Emiliano NF, Martins MT, Souza SS, de Souza AM, Berto C Jr, Costa ML, Campos LM, França FD, Tagliati CA.Toxicological in vitro and subchronic evaluation of LASSBio-596.Food and Chemical Toxicology, v. 73, p. 148-156, 2014.[DOI]
17.Carvalho GMC, Oliveira VR, Gomes MDM, Casquilho NV ,Araujo AP, Soares RM , Azevedo SO, Monteiro APT , Claudio Canetti C , Lima LM , Barreiro EJ, Zin WA . Pulmonary And Hepatic Injuries Caused By TheSubchronic Exposure To Microcystin-lr: Role Of The Duration Of Treatment With LASSBio 596. Seminars in Respiratory and Critical Care Medicine, v. 189, p. A5023-A5023, 2014.[LINK]
18. Sampaio TS, Lima LM, Zardo RS, Pessoa C, Cavalcanti BC, Castro RP, Sabino JR, Fernandes PD, Barreiro EJ. Synthesis, Antiproliferative and Anti-inflammatory Activities of Novel Simplified Imatinib Analogues.Medicinal Chemistry, v. 4, p. 756-762, 2014.[DOI]
19.Chagas-Silva F, Nascimento-Viana JB, Romeiro LAS, Barberato LC, Noël F, Silva CLM. Pharmacological characterization of N1-(2-methoxyphenyl)-N4-hexylpiperazine as a multi-target antagonist of α1A/α1D-adrenoceptors and 5-HT1A receptors that blocks prostate contraction and cell growth.Naunyn-Schmiedeberg's Archives of Pharmacology, v. 387, p. 225-234, 2014.[DOI]
20. Noël F, Pompeu TET, Moura, BC. Functional binding assays for estimation of the intrinsic efficacy of ligands at the 5-HT1A receptor: Application for screening drug candidates. Journal of Pharmacological and Toxicological Methods, v. 70, p. 12-18, 2014.[DOI]
21. Stolz ED, Müller LG, Antonio CB, da Costa PF, Von Poser GL, Noël F, Rates SMK.Determination of pharmacological interactions of uliginosin B, a natural phloroglucinol derivative, with amitriptyline, clonidine and morphine by isobolographic analysis.Phytomedicine (Stuttgart), v. 21, p. 1684-1688, 2014.[DOI]
22. Rocha SC, Pessoa MTC,Neves LDR, Alves SLG, Silva LM, Santos HL, Oliveira SMF, Taranto AG,Comar M, Gomes IV, Santos FV, Paixão N, Quintas LEM, Noël F, Pereira AF, , Tessis ACSC, , Gomes NLS, Moreira OC, Rincon-Heredia RK, Varotti FP, Blanco G,Villar JAPF, Contreras RG, Barbosa LA.21-Benzylidene Digoxin: A ProapoptoticCardenolide of Cancer Cells That Up-Regulates Na,K-ATPase and Epithelial Tight Junctions. Plos One, v. 9, p. e108776, 2014.[DOI]
23. Cavalcante-Silva LHA, Falcão MAP, Vieira ACS, Viana MDM, de Araújo-Júnior JX, Sousa JCF, da Silva TMS, Barbosa-Filho JM, Noël F, de Miranda GEC, de Oliveira Santos BV, Alexandre-Moreira MS.Assessment of Mechanisms Involved in Antinociception Produced by the Alkaloid Caulerpine. Molecules (Basel. Online), v. 19, p. 14699-14709, 2014.[DOI]
24. Zapata-Sudo G, da Silva JS, Pereira SL, Souza PJC, de Moura RS, Sudo RT. Oral treatment with Euterpe oleracea Mart. (açaí) extract improves cardiac dysfunction and exercise intolerance in rats subjected to myocardial infarction. BMC ComplementaryandAlternative Medicine (Online), v. 14, p. 227, 2014. [DOI]
25. Debom RC, Trachez MM, Zapata-Sudo G, da Silva JS, Oliveira KM, Garcia AL, Godoy LM, Pacheco PC, Sudo RT.Novel Nicotinic Receptor Agonist Reduces Hyperalgesia and Allodynia of Neuropathic Pain in Diabetic Rats. Journalof Diabetes &Metabolism, v. 5, p. 396, 2014.[DOI]
26. Zapata-Sudo G, Sudo SZ, Alencar AKN, Sudo RT. Targeting of the Adenosine Receptors as a Novel Strategy for the Treatment of Arterial Hypertension. Journal of Neurology and Neurophysiology, v. 5, p. 243, 2014.[LINK]
27. Alencar AKN, Barreiro EJ,Sudo RT,Zapata-Sudo G. Adenosine A2A Receptor as a Target of Treatment for Pulmonary Arterial Hypertension.ClinicalResearch in Pulmonology, v. 2, p. 1021, 2014.[LINK]
28. deOliveira GP, Silva JD, de Araújo CC, Prota LFM, Abreu SC, Madeira C, Morales MM, Takiya CM, Diaz BL, Capelozzi VL, Panizzutti R, Pelosi P, Rocco PRM. Intravenous Glutamine Administration Reduces Lung and Distal Organ Injury in Malnourished Rats With Sepsis. Shock,v. 41, p. 222 - 232, 2014.[DOI]
29. Saddy F, Sutherasan Y, Rocco PR, Pelosi P.Ventilator-Associated Lung Injury during Assisted Mechanical Ventilation. Seminars in Respiratory and Critical Care Medicine, v. 35, p. 409-417, 2014.[DOI]
30. Silva JD, Paredes BD, Araújo IM, Lopes-Pacheco M, Oliveira MV, Suhett GD, Faccioli LA, Assis E, Castro-Faria-Neto HC, Goldenberg RC, Capelozzi VL, Morales MM, Pelosi P, Xisto DG, Rocco PRM.Effects of bone marrow derived mononuclear cells from healthy or acute respiratory distress syndrome donors on recipient lung-injured mice. CriticalCare Medicine, v. 42, p. e510-524, 2014.[DOI]
31. Burburan SM, Silva JD, Abreu SC, Samary CS, Guimarães IH, Xisto DG, Morales MM, Rocco PRM. Effects of inhalational anaesthetics in experimental allergic asthma.Anaesthesia (London.Print), v. 6, p. 573-582, 2014.[DOI]
32. Elias ASNT, Oliveira GP, Ornellas DS, Morales MM, Capelozzi VL, Haddad R, Pelosi P, Rocco PRM, Garcia CSNB. Effects of early and late pneumothorax drainage on the development of pulmonary oedema.Respiratory Physiology & Neurobiology, v. 195, p. 27-36, 2014.[DOI]
33. Krebs J, Tsagogiorgas C, Pelosi P, Rocco PRM, Hottenrott M , Sticht C , Yard B, Luecke T. Open lung approach with low tidal volume mechanical ventilation attenuates lung injury in rats with massive brain damage. Critical Care (London. Print), v. 18, p. R59, 2014.[DOI]
34. da Silva AL, Martini SV, Abreu SC, Samary CDS, Diaz BL, Fernezlian S, de Sá VK, Capelozzi VL, Boylan NJ, Goya RG, Suk, JS, Rocco PRM, Hanes J, Morales MM.DNA nanoparticle-mediated thymulin gene therapy prevents airway remodeling in experimental allergic asthma. Journal of Controlled Release, v. 180, p. 125-133, 2014.[DOI]
35. Uhlig C, Silva PL, Ornellas D, Santos RS, MirandaPJ, Spieth PM, Kiss T, Kasper M, Wiedemann B, Koch T, Morales MM, Pelosi P, de Abreu MG, Rocco PRM. The effects of salbutamol on epithelial ion channels depend on the etiology of acute respiratory distress syndrome but not the route of administration. RespiratoryResearch (Print), v. 15, p. 56, 2014.[DOI]
36. Santos CL, Moraes L, Santos RS, dos Santos SC, Silva JD, Morales MM, Capelozzi VL, de Abreu MG,Schanaider A, Silva PL, Garcia CSNB,Pelosi P, Rocco PRM. The biological effects of higher and lower positive end-expiratory pressure in pulmonary and extrapulmonary acute lung injury with intra-abdominal hypertension. Critical Care (London. Print), v. 18, p. R121, 2014.[DOI]
37. Antunes MA, Laffey JG, Pelosi P, Rocco PRM. Mesenchymal Stem Cell Trials for Pulmonary Diseases.Journal of Cellular Biochemistry (Print), v. 115, p. 1023-1032, 2014.[DOI]
38. Silva PL, Pelosi P, Rocco PRM.Fluids in acute respiratory distress syndrome.CurrentOpinion in Critical Care, v. 20, p. 104-112, 2014.[DOI]
39. Martini SV, da Silva AL, Ferreira D, Gomes K, Ornellas FM, Lopes-Pacheco M, Zin E, Petrs-Silva H, Rocco PRM, Morales MM. Single Tyrosine Mutation in AAV8 Vector Capsid Enhances Gene Lung Delivery and Does Not Alter Lung Morphofunction in Mice. Cellular Physiology and Biochemistry (Online), v. 34, p. 681-690, 2014.[DOI]
40. Moraes L, Santos CL, Santos RS, Cruz FF, Saddy F, Morales MM, Capelozzi VL, Silva PL, de Abreu MG , Garcia CSNB, Pelosi P, Rocco PRM. Effects of sigh during pressure control and pressure support ventilation in pulmonary and extrapulmonary mild acute lung injury. Critical Care (London. Print), v. 18, p. 474, 2014.[DOI]
41. Rodríguez-González R, Ramos-Nuez Á, Martín-Barrasa JL, López-Aguilar J, Baluja A, Álvarez J, Rocco PRM, Pelosi P, Villar J.Endotoxin-induced lung alveolar cell injury causes brain cell damage. Experimental Biology and Medicine (Maywood, N.J.: Print), v. 240, p. 135-142, 2014.[DOI]
42. Abreu SC, Antunes MA, Mendonça L, Branco VC, de Melo EB, Olsen PC, Diaz BL, Weiss DJ, Paredes BD , Xisto DG, Morales MM, Rocco PRM. Effects of bone marrow mononuclear cells from healthy or ovalbumin-induced lung inflammation donors on recipient allergic asthma mice. Stem Cell Research &Therapy, v. 5, p. 108-108, 2014.[DOI]
43. Spieth PM, Silva PL, Garcia CSNB, Ornellas DS, Samary CS, Moraes L, Bentes M, Morales MM, Kasper M, Güldner A, Huhle R, Koch T, Pelosi P, de Abreu MG, Rocco PRM. Modulation of Stress versus Time Product during Mechanical Ventilation Influences Inflammation as Well as Alveolar Epithelial and Endothelial Response in Rats.Anesthesiology (Philadelphia), v. 122, p. 106-116, 2014.[DOI]
44.Carvalho NC, Güldner A, Beda A, Rentzsch I, Uhlig C, Dittrich S, Spieth PM, Wiedemann B, Kasper M, Koch T, Richter T, Rocco PRM, Pelosi P, de Abreu MG.Higher Levels of Spontaneous Breathing Reduce Lung Injury in Experimental Moderate Acute Respiratory Distress Syndrome. CriticalCare Medicine, v. 42, p. e702-715, 2014.[DOI]
45. Carvalho GM, Nagato LK, Fagundes Sda S, Dos Santos FB, Calheiros AS, Malm O, Bozza PT, Saldiva PH, Faffe DS, Rocco PRM, Zin WA.Time course of pulmonary burden in mice exposed to residual oil fly ash. Frontiers in Physiology, v. 5, p. 366-366, 2014.[LINK]
46. Kuhl CP, Garcez TNA, Magrisso AB, Laurino CCFC, Cirne-Lima EO, Rocco PRM, Macedo-Neto AV.Development of different degrees of elastase-induced emphysema in mice: a randomized controlled experimental study. Clinical and Biomedical Research, v. 34, p. 297-306, 2014.[LINK]
47.Nardelli L.M, Rzezinski A, Silva JD, Maron-Gutierrez T, Ornellas DS, Henriques LI, Capelozzi VL, Teodoro WR, Morales MM, Silva PL, Pelosi P, Garcia CSNB, Rocco PRM. Effects Of Acute Hypercapnia With And Without Acidosis On Lung Inflammation And Apoptosis In Experimental Acute Lung Injury. Respiratory Physiology & Neurobiology, v.205, p. 1- 6, 2014.[DOI]
48. Cavalcanti V, Santos CL, Samary CS, Araújo MN, Heil LBB, Morales MM, Silva PL, Pelosi P, Fernandes FC,VillelaN, Rocco PRM. Effects of short-term propofol and dexmedetomidine on pulmonary morphofunction and biological markers in experimental mild acute lung injury.Respiratory Physiology & Neurobiology, v. 203, p. 45-50, 2014.[DOI]
49. Antunes MA, Abreu SC, Cruz FF, Teixeira AC, Lopes-Pacheco M, Bandeira E, Olsen PC, Diaz BL, Takyia CM, Freitas IPRG, Rocha NN,Capelozzi VL, Xisto DG, Weiss DJ, Morales MM, Rocco PRM. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema.RespiratoryResearch (Print), v. 15, p. 118, 2014.[DOI]
50.Lopes-Pacheco M, Ventura TG, de Oliveira HD, Monção-Ribeiro LC, GutfilenB, de Souza SAL, Rocco PRM, Borojevic R, Morales MM, Takiya CM. Infusion of bone marrow mononuclear-cells reduces lung fibrosis but not inflammation in the late stages of murine silicosis. Plos One, v. 9, p. e109982, 2014.[DOI]
51. Lee JW, Rocco PRM, Pelosi P.Mesenchymal Stem Cell Therapy for Acute Respiratory Distress Syndrome. Anesthesiology (Philadelphia), v. 122, p. 238-240, 2014.[DOI]
52. Pelosi P, Rocco PRM. To prevent or cure acute respiratory distress syndrome: That is the question! Current Opinion in Critical Care.v.20, p. 1-2, 2014.[DOI]
53. Maron-Gutierrez T, Laffey JG, Pelosi P, Rocco PRM. Cell-based therapies for the acute respiratory distress syndrome.Current Opinion in Critical Care, v.20, p. 122-131, 2014.[DOI]
54. Righetti RF, Pigati PADS, Possa SS, , Habrum FC, Xisto DG, Antunes MA, , Leick EA, Prado CM, Martins MDA, Rocco PRM, Tibério IDFLC. Effects of Rho-kinase inhibition in lung tissue with chronic inflammation.Respiratory Physiology and Neurobiology, v. 192, p.134-146, 2014.[DOI]
55. Coelho-Cerqueira E, Netz PA, do Canto VP, Pinto AC, Follmer C. Beyond Topoisomerase Inhibition: Antitumor 1,4-Naphthoquinones as Potential Inhibitors of Human Monoamine Oxidase. Chemical Biology & Drug Design (Print), v. 83, p. 401-410, 2014.[DOI]
56. Martinez ST, Pinto AC, Glasnov T, Kappe CO. Chemistry of pyrrolizidine alkaloids revisited-semi-synthetic microwave and continuous-flow approaches toward Crotalaria-alkaloids. Tetrahedron Letters, v. 55, p. 4181-4184, 2014.[DOI]
57. Boechat N, Pinheiro LCS, Pinto AC, Silva TS, Waedell J, Wardell, SMSV. Crystal structures of two anhydrous and one hydrated 7-(arylamino)-5-methyl-2-(trifluoromethyl)-[1,2,4]-triazolo-[1,5-a]pyrimidine derivatives. Zeitschrift fur Kristallographie, v. 229, p. 459-471, 2014.[LINK]
58. Fierro IM, de Menezes Alencar MS, Lins Mendes FM, de Souza Mendes C, Nunes BF, Antunes AMS.Nanoparticles applied to antineoplastic agents: a patent landscape. PharmaceuticalPatentAnalyst, v. 3, p. 613-623, 2014.[DOI]
59. Ribeiro-Filho J, Leite FC, Costa HF, Calheiros AS, Torres RC, de Azevedo CT, Martins MA, Dias CdaS, Bozza PT, Piuvezam MR. Curine inhibits mast cell-dependent responses in mice. Journal of Ethnopharmacology, v. 155, p. 1118-1124, 2014.[DOI]
60. Nihi F, Moreira D, Santos Lourenço AC, Gomes C, Araujo SL, Zaia RM, Trevisani NB, Pinto LD, Moura-Costa DD, de Morais RN, Paumgartten FJR, Martino-Andrade AJ.Testicular Effects Following In Utero Exposure to the Antivirals Acyclovir and Ganciclovir in Rats. Toxicological Sciences (Print), v. 139, p. 220-233, 2014.[DOI]
61. Hussin M,SitiAmrah S,Hasnan J,Paumgartten FJR.Evaluation on the reproductive performance and spontaneous malformations amongst SD rats in the Institute for Medical Research colony. Malaysian Journal of Veterinary Research, v.5,p. 53-63, 2014.[Link]
62. Paumgartten FJR, de Oliveira ACAX. The bisphenolA toxicological paradox: The more we learn the less we know for sure.Environmental SkepticsandCritics, v. 3, p. 65-82, 2014.[LINK]
63. Coelho DR,de Carvalho RR, Rocha RCC,Saint'Pierre TD,Paumgartten FJR. Effects of in utero and lactational exposure to SbV on rat neurobehavioral development and fertility.ReproductiveToxicology (Elmsford, N.Y.), v. 50, p. 98-107, 2014.[DOI]
64. Rocha MRE, de Souza JJ, Barcellos LT, Sant'Anna CMR, Braz-Filho R, Vieira IJC. A Novel 3,9-(1,2,3-Trioxocine)-Type Steroid of Rauianodosa (Rutaceae). Molecules (Basel.Online), v. 19, p. 14637-14648, 2014. [DOI]
65. de Magalhães CS, Almeida DM, Barbosa HJC,Dardenne LE.A dynamic niching genetic algorithm strategy for docking highly flexible ligands.Information Sciences, v. 289, p. 206-224, 2014.[DOI]
66. Custódio FL, Barbosa HJC, Dardenne LE. A multiple minima genetic algorithm for protein structure prediction.Applied Soft Computing (Print), v. 15, p. 88-99, 2014.[DOI]
67. Guedes IA, de Magalhães CS, Dardenne LE. Receptor-ligand molecular docking.Biophysical Reviews, v. 6, p. 75-87, 2014.[DOI]
68. Vieira AM, Neto EH, Figueiredo CC, Barja- Fidalgo C, Fierro IM, Morandi V. ATL-1, a synthetic analog of lipoxin, modulates endothelial permeability and interaction with tumor cells through a VEGF-dependent mechanism.Biochemical Pharmacology, v. 90, p. 388-396, 2014.[DOI]
69. Ribeiro-Pereira C, Moraes JA, Souza Mde J, Laurindo FR, Arruda MA, Barja-Fidalgo C. Redox modulation of FAK controls melanoma survival - Role of NOX4. Plos One, v. 9, p. e99481, 2014.[DOI]
70. Boa BCS, Souza MGC, Leite RD, da Silva SV, Barja-Fidalgo TC,Kraemer-Aguiar LG,, Bouskela E. Chronic Aerobic Exercise Associated to Dietary Modification Improve Endothelial Function and eNOS Expression in High Fat Fed Hamsters. Plos One, v. 9, p. e102554, 2014.[DOI]
71. Bueno RV, Braga RC,SegrettiND, Ferreira EI, Trossini GHG, Andrade CH. New Tuberculostatic Agents Targeting Nucleic Acid Biosynthesis: Drug Design using QSAR Approaches. CurrentPharmaceutical Design (Print), v. 20, p.4474-4485,2014. [LINK]
72. Serafim RAM, Gonçalves JE, de Souza FP, Loureiro APM, Storpirtis S, Krogh R, Andricopulo AD, Dias LC, Ferreira EI. Design, synthesis and biological evaluation of hybrid bioisoster derivatives of N-acylhydrazone and furoxan groups with potential and selective anti-Trypanosomacruzi activity.European Journal of Medicinal Chemistry, v. 82, p. 418-425, 2014.[DOI]
73. Lima-Júnior RC, Freitas HC, Wong DV, Wanderley CW, Nunes LG, Leite LL, Miranda SP, Souza MH, Brito GA, Magalhães PJ, Teixeira MM, Cunha FQ, Ribeiro RA. Targeted Inhibition of Interleukin-18 Attenuates Irinotecan-Induced Intestinal Mucositis in Mice. British JournalofPharmacology, v. 171, p. 2335 – 2350, 2014.[DOI]
74. Ferreira AE, Sisti F, Sônego F, Wang S, Filgueiras LR, Brandt S, Serezani AP, Du H, Cunha FQ, Alves-Filho JC, Serezani CH.PPAR- /IL-10 Axis Inhibits MyD88 Expression and Ameliorates Murine Polymicrobial Sepsis. Journal of Immunology, v. 192, p. 2357-2365, 2014.[DOI]
75. Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS, Braun H, Lima-Junior RC, Ribeiro RA, Cunha FQ, Teixeira MM, Beyaert R, Graham GJ, Liew FY. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. MucosalImmunology, v.7,p. 1079 – 1093, 2014.[DOI]
76. Hernandes MS, D'Avila JC, Trevelin SC, Reis PA, Kinjo ER, Lopes LR, Castro-Faria-Neto HC, Cunha FQ, Britto LR, Bozza FA.The role of Nox2-derived ROS in the development of cognitive impairment after sepsis.Journal of Neuroinflammation, v. 11, p. 36-48, 2014.[DOI]
77. Vieira SM, Silva RL, Lemos HP, Amorim RC, Silva EC, Reinach PS, Cunha FQ, Pohlit AM, Cunha TM.Gastro-protective effects of isobrucein B, a quassinoid isolated from Picrolemmasprucei.Fitoterapia,v. 95, p. 8-15, 2014.[DOI]
78. de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FA, Pruijn GJ, Marijnissen RJ, Walgreen B, Helsen MM, van denBersselaar LA, de Molon RS, Avila Campos MJ, Cunha FQ, Cirelli JA, van den Berg WB.Periodontal Pathogens Directly Promote Autoimmune Experimental Arthritis by Inducing a TLR2- and IL-1-Driven Th17 Response. Journal of Immunology, v. 192, p. 4103 – 4111, 2014.[DOI]
79. Wanderley CW, Silva CM, Wong DV, Ximenes RM, Morelo DF, Cosker F, Aragão KS, Fernandes C, Palheta-Júnior RC, Havt A, Brito GA, Cunha FQ, Ribeiro RA, Lima-Júnior RC. Bothropsjararacussu snake venom-induces a local inflammatory response in a prostanoid- and neutrophil-dependent manner. Toxicon (Oxford), v. 90, p. 134 - 147, 2014.[DOI]
80. Saia RS, Mestriner FL, Bertozi G, Cunha FQ, Cárnio EC. Cholecystokinin Inhibits Inducible Nitric Oxide Synthase Expression by Lipopolysaccharide-Stimulated Peritoneal Macrophages. MediatorsofInflammation (Print), v. 2014, p. 1-14, 2014.[DOI]
81. Sônego F, Castanheira FVS, Czaikoski PG, Kanashiro A, Souto FO, França RO, Nascimento DC, Freitas A, Spiller F, Cunha LD, Zamboni DS, Alves-Filho JC, Cunha FQ. MyD88-, but Not Nod1- and/or Nod2-Deficient Mice, Show Increased Susceptibility to Polymicrobial Sepsis due to Impaired Local Inflammatory Response. PlosOne, v. 9, e103734, 2014.[DOI]
82. Staurengo-Ferrari L, Zarpelon AC, Longhi-Balbinot DT, Marchesi M, Cunha TM, Alves-Filho JC, Cunha FQ, Ferreira SH, Casagrande R, Miranda KM, Verri WA Jr.Nitroxyl inhibits overt pain-like behavior in mice: Role of cGMP/PKG/ATP-sensitive potassium channel signaling pathway. Pharmacological Reports, v. 66, p. 691-698, 2014.[DOI]
83. Barragán-Iglesias P, Rocha-González HI, Pineda-Farias JB, Murbartián J, Godínez-Chaparro B, Reinach PS, Cunha TM, Cunha FQ, Granados-Soto V. Inhibitionofperipheralanionexchanger 3 decreasesformalin-inducedpain. European Journal of Pharmacology, v. 738, p. 91-100, 2014.[DOI]
84. Sônego F, Alves-Filho JC, Cunha FQ. Targeting neutrophils in sepsis.Expert ReviewofClinicalImmunology, v. 10, p. 1019 - 1028, 2014.[DOI]
85. Borghi SM, Zarpelon AC, Pinho-Ribeiro FA, Cardoso RDR, Cunha TM, Alves-Filho JC, Ferreira SH, Cunha FQ, Casagrande R, Verri WA. Targeting interleukin-1β reduces intense acute swimming-induced muscle mechanical hyperalgesia in mice. Journal of Pharmacy and Pharmacology, v. 66, p. 1009-1020, 2014.[DOI]
86. Calil IL, Zarpelon AC, Guerreiro ATG, Alves-Filho JC, Ferreira SH, Cunha FQ, Cunha TM, Verri WA Jr. Lipopolysaccharide Induces Inflammatory Hyperalgesia Triggering a TLR4/MyD88-Dependent Cytokine Cascade in the Mice Paw. PlosOne, v.9, p. e90013 2014.[DOI]
87. Borghi SM, Zarpelon AC, Pinho-Ribeiro FA, Cardoso RD, Martins-Pinge MC, Tatakihara RI, Cunha TM, Ferreira SH, Cunha FQ, Casagrande R, Verri WA Jr.Role of TNF-α/TNFR1 in intense acute swimming-induced delayed onset muscle soreness in mice. Physiology&Behavior, v. 128, p. 277-287, 2014.[DOI]
88. de Melo L AC, Alves TM, Carneiro GVC, de Lima PMM, de Melo NR, Cunha TM, Pinto ACMD, Cunha FQ, Rocha FAC.Meniscal transection rather than excision increases pain behaviour and structural damage in experimental osteoarthritis in mice. OsteoarthritisandCartilage, v.22, p. 1878-1985, 2014.[DOI]
89. dos Reis Barbosa AL, de Sousa RB, Torres JN, Cunha TM, de Queiroz Cunha F, Soares PM, de Albuquerque Ribeiro R, Vale ML, Souza MH.Colitis generates remote antinociception in rats: the role of the l-arginine/NO/cGMP/PKG/KATP pathway and involvement of cannabinoid and opioid systems. Inflammation Research, v. 63, p. 969 – 977, 2014.[DOI]
90. Moriconi A, Cunha TM, Souza GR, Lopes AH, Cunha FQ, Carneiro VL, Pinto LG, Brandolini L, Aramimi A, Bizzarri C, Bianchini G, Beccari AR, Fanton M, Bruno A, Costantino G, Bertini R, Galliera E, Locati M, Ferreira SH, Texeira MM, Allegretti M. Targeting the minor pocket of C5aR for the rational design of an oral allosteric inhibitor for inflammatory and neuropathic pain relief. Proceedings of the National Academy of Sciences of the United States of America, v.111, P.16937–16942, 2014.[DOI]
91. Sacramento LA, Cunha FQ, de Almeida RP, da Silva JS, Carregaro V. Protective Role of 5-Lipoxigenase duringLeishmaniainfantumInfectionIs Associated with Th17 Subset. BioMedResearchInternational, v. 2014, p. 264-270, 2014.[DOI]
92. Prates TP, Taira TM, Holanda MC, Bignardi LA, Salvador SL, Zamboni DS, Cunha FQ, Fukada SY. NOD2 Contributes to Porphyromonasgingivalis-induced Bone Resorption. Journal of Dental Research (Online), v. 93, p. 1155-1162, 2014.[DOI]
93. Gubiani JR, Zeraik ML, Oliveira CM, Ximenes VF, Nogueira CR, Fonseca LM, Silva DH, Bolzani VS, Araujo AR.Biologically Active Eremophilane-Type Sesquiterpenes from Camarops sp., an Endophytic Fungus Isolated from Alibertiamacrophylla. Journal of Natural Products (Print), v. 77, p. 668-672, 2014.[DOI]
94. Queiroz MMF, Queiroz EF, Zeraik ML, Ebrahimi SN, Marcourt L, Cuendet M, Castro-Gamboa I, Hamburger M, Bolzani VS, Wolfender JL. Chemical Composition of the Bark of Tetrapterysmucronata and Identification of Acetylcholinesterase Inhibitory Constituents.Journal of Natural Products (Print), v. 77, p. 650–656, 2014.[DOI]
95. Chapla VM, Zeraik ML, Ximenes VF, Zanardi LM, Lopes MN, Cavalheiro , Silva DH, Young MC, Fonseca LM, Bolzani VS, Araújo AR. Bioactive Secondary Metabolites from Phomopsis sp., an Endophytic Fungus from Senna spectabilis. Molecules (Basel.Online), v. 19, p. 6597-6608, 2014.[DOI]
96. Altei WF, Picchi DG, Abissi BM, Giesel GM, Flausino O Jr, Reboud-Ravaux M, Verli H, Crusca E Jr, Silveira ER, Cilli EM, Bolzani VS. Jatrophidin I, a cyclic peptide from Brazilian Jatropha curcas L.: Isolation, characterization, conformational studies and biological activity. Phytochemistry, v. 107, p. 91-96, 2014.[DOI]
97. Coqueiro A, Regasini LO, Stapleton P, da Silva Bolzani V, Gibbons S. In Vitro Antibacterial Activity of Prenylated Guanidine Alkaloids from and Synthetic Analogues.Journal of Natural Products (Print), v. 77, p. 1972-1975, 2014.[DOI]
98. Ferreira RS, Dessoy MA, Pauli I, Souza ML, Krogh R, Sales AI, Oliva G, Dias LC, Andricopulo AD. Synthesis, Biological Evaluation, and Structure-Activity Relationships of Potent Noncovalent and NonpeptidicCruzain Inhibitors as Anti- Trypanosomacruzi Agents.Journal of Medicinal Chemistry, v. 57, p. 2380-2392, 2014.[DOI]
99. Campagne JM, de Figueiredo RM, Suppo JS, de Sant’ana DP, Dias LC. Efficient and Practical Procedure for the Esterification of the Free α-Carboxylic Acid of Amino Acid Residues with β-(Trimethylsilyl)ethoxymethyl Chloride and Triisopropylsilyl Chloride. Synthesis (Stuttgart), v. 46, p. 3075-3084, 2014.[DOI]
100. Gomes LMF, Vieira RP, Jones MR, Wang MCP, Dyrager C, Souza-Fagundes EM, da SilvaJG, Storr T, Beraldo H. 8-Hydroxyquinoline Schiff-base Compounds as Antioxidants and Modulators of Copper-Mediated Aβ Peptide Aggregation. Journal of Inorganic Biochemistry, v. 139, p. 106-116, 2014.[DOI]
101. Islam A, da Silva JG, Berbet FM, da Silva SM, Rodrigues BL, Beraldo H, Melo MN, Frézard F, Demicheli C. Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages. Molecules (Basel. Online), v. 19, p. 6009-6030, 2014. [DOI]
102. Parrilha GL, Ferraz KSO, Lessa JÁ, de Oliveira KN, Rodrigues BL, Ramos JP, Souza-Fagundes EM, Ott I, Beraldo H. Metal complexes with 2-acetylpyridine-N(4)-orthochlorophenylthiosemicarbazone: cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. European Journal of Medicinal Chemistry, v. 84, p. 537-544, 2014.[DOI]
103. Despaigne AAR, da Silva JG, da Costa PR, dos Santos RG, Beraldo H. ROS-Mediated Cytotoxic Effect of Copper(II) Hydrazone Complexes against Human Glioma Cells. Molecules (Basel. Online), v. 19, p. 17202-17220, 2014. [DOI]
104. Dias KS, Viegas Jr. C. Multi-Target Directed Drugs: A Modern Approach for Design of New Drugs for the treatment of Alzheimer-s Disease. Current Neuropharmacology, v. 12, p. 239-255, 2014.[DOI]
105. Simões MCR, Viegas FPD, Moreira MS, Silva MF, Riquiel MM, da Rosa PM, Castelli MR, dos Santos MH, Soares MG, Viegas Jr. C. Donepezil: An Important Prototype to the Design of New Drug Candidates for Alzheimer s Disease. Mini-Reviews in Medicinal Chemistry, v. 14, p. 2-19, 2014.[DOI]
106. Pivatto M, Baccinib LR, Sharmac A, Nakabashic M, Danuello A, Viegas Jr. C, Garcia CRS, Bolzani VS. Antimalarial activity of piperidine alkaloids from Senna spectabilis and semisynthetic derivatives.Journal of the Brazilian Chemical Society, v. 25, p. 1900-1906,2014.[DOI]
107. de Albuquerque Melo GM, Silva MC, Guimarães TP, Pinheiro KM, da Matta CB, de Queiroz AC, Pivatto M, BolzaniVda S, Moreira MS, Viegas Jr. C. Leishmanicidal activity of the crude extract, fractions and major piperidine alkaloids from the flowers of Senna spectabilis. Phytomedicine (Stuttgart), v. 21, p. 277-281, 2014. [DOI]
108. Moreira MEC, PereiraRGF, Dias Silva MJ, Dias DF, Gontijo VS, Giusti-Paiva A, Veloso MP,Doriguetto AC, Nagem TJ, dos Santos MH.Analgesic and Anti-Inflammatory Activities of the 2,8-Dihydroxy-1,6-Dimethoxyxanthone from Haploclathrapaniculata (Mart) Benth (Guttiferae). Journal of Medicinal Food. v. 17, p. 686-693, 2014.[DOI]
109. Centurião FB, Braga A, Machado FR, Tagliari B, Müller LG, Kolling J, Von Poser G, Wyse ATS, Rates SMK. Study of antidepressant-like activity of an enriched phloroglucinol fraction obtained from Hypericumcaprifoliatum.Pharmaceutical Biology.v. 52,p. 105-110, 2014. [DOI]
110. Centurião FB, Sakamoto S, Stein AC, Müller LG, Chagas PM, Poser GLV, Nogueira CW, Rates SMK. The Antidepressant-like Effect of Hyperbrasilol B, A Natural Dimeric Phloroglucinol Derivative is prevented by Veratrine, A Sensitive-Voltage Na+ Channel Opener. European Journal of Medicinal Plants, v. 4, Issue 11, p. 1268-1281,2014.[DOI]
111. Duarte MO, Lunardelli S, Kiekow CJ, Stein AC, Mul̈ler L, Stolz E, Rates SMK, Gosmann G. Phloroglucinol derivatives present an antidepressant-like effect in the mice tail suspension test (TST). Natural Product Communications, v. 9, p. 671-674, 2014.[LINK]
112. Stolz ED, Hasse DR, Von Poser GL, Rates SMK. Uliginosin B, a natural phloroglucinol derivative, presents a multimediatedantinociceptive effect in mice. Journal of Pharmacy and Pharmacology, v. 66, p. 1774-1785, 2014.[DOI]
113. Stolz ED, Müller LG, Antonio CB, da Costa PF, Von Poser GL, Noël F, Rates SMK. Determination of pharmacological interactions of uliginosin B, a natural phloroglucinol derivative, with amitriptyline, clonidine and morphine by isobolographic analysis.Phytomedicine (Stuttgart), v. 21, p. 1684-1688, 2014.[DOI]
114. Ccana-Ccapatinta GV, Stolz ED, da Costa PF, Rates SMK, Von Poser GL. Acylphloroglucinol Derivatives from : Antidepressant-like Activity of Andinin A. Journal of Natural Products (Print), v. 77, p. 2321-2325, 2014.[DOI]
115. Borsoi M, Antonio CB, Viana AF, Nardin P, Gonçalves CA, Rates SMK. Immobility behavior during the forced swim test correlates with BNDF levels in the frontal cortex, but not with cognitive impairments.Physiology and Behavior.v.140, p. 79-88, 2014.
116. Torres BG, UchôaFde T, Pigatto MC, Azeredo FJ, Haas SE, Dallegrave E, Canto RF, Eifler-Lima VL, Dalla Costa T. Pre-Clinical Pharmacokinetics and Acute Toxicological Evaluation of a Monastrol Derivative Anticancer Candidate LaSOM 65 in Rats. Xenobiotica, v. 44, p. 254-263, 2014.[DOI]
117. Haas SE, de Andrade C, da Silva Sansone PE, GuterresS ,Dalla Costa T. Development of innovative oil-core self-organized nanovesicles prepared with chitosan and lecithin using a 2x2x2 full-factorial design. Pharmaceutical Development and Technology, v. 19, p. 769-778, 2014.[DOI]
118. de Andrade C, de Araújo Lock G, Pigatto MC, Haas SE, Costa TD, de Araújo BV.Validation of LC-MS/MS method applied to evaluation of free tissue concentrations of vildagliptin in diabetic rats by microdialysis. BMC.Biomedical Chromatography, v. 28, p.1722-1727, 2014.[DOI]
119. Penso J, Cordeiro KC, da Cunha CR, da Silva Castro PF, Martins DR, Lião LM, Rocha ML, de Oliveira V. Vasorelaxantactivityof 7-β-O-glycosidesbiosynthesizedfromflavonoids. European Journal of Pharmacology, v. 733, p. 75-80, 2014.[DOI]
120. Fajemiroye JO, Amaral NO, da Silva EF, Galdino PM, de Oliveira TS, Ghedini PC, Zjawiony JK, Costa EA, Pedrino GR, Menegatti R. Hypotensive and Antihypertensive Potential of 4-[(1-Phenyl-1H-Pyrazol-4-yl) Methyl]1-Piperazine Carboxylic Acid Ethyl Ester: A Piperazine Derivative. Life Sciences, v. 112, p. 90-96, 2014.[DOI]
121. Menezes JB, da Silva T, dos Santos J, Catari E, Meneghetti M, da Matta CB, Alexandre-Moreira MS, Santos-Magalães N, Grillo L, Dornelasa C.Layered double hydroxides (LDHs) as carrier of antimony aimed for improving leishmaniasis chemotherapy. Applied Clay Science, v. 91-92, p. 127-134, 2014.[DOI]
122. de Queiroz AC, Alves Hda S, Cavalcante-Silva LH, Dias Tde L, Santos Mda S, Melo GM, Campesatto EA, Chaves MC, Alexandre-Moreira MS.Antinociceptive and anti-inflammatory effects of flavonoids PMT1 and PMT2 isolated from Piper montealegreanumYuncker (Piperaceae) in mice. Natural ProductResearch, v.28,p. 403 – 406,2014.[DOI]
123. de Queiroz AC, Dias T de L, da Matta CB, Silva LHAC, Araújo-Júnior JX, Araújo GB, Moura FBP, Alexandre-Moreira MS.Antileishmanial Activity of Medicinal Plants Used in Endemic Areas in Northeastern Brazil. Evidence-Based Complementary and Alternative Medicine, v. 2014, p. 1-9, 2014.[DOI]
124. Cavalcante-Silva LHA, Falcão MAP, Vieira ACS, Viana MDM, de Araújo-Júnior JX, Sousa JCF, da Silva TMS, Barbosa-Filho JM, Noël F, de Miranda GEC, de Oliveira Santos BV, Alexandre-Moreira MS. Assessment of Mechanisms Involved in Antinociception Produced by the Alkaloid Caulerpine. Molecules, v. 19, p. 14699-14709, 2014.[DOI]
125. de Araújo MV, de Souza PS, de Queiroz AC, da Matta CB, Leite AB, da Silva AE, de França JA, Silva TM, Camara CA, Alexandre-Moreira MS. Synthesis, Leishmanicidal Activity and Theoretical Evaluations of a Series of Substituted bis-2-Hydroxy-1,4-Naphthoquinones. Molecules, v. 19, p. 15180-15195, 2014.[DOI]
126. Morano MT, Mesquita R, Da Silva GP, Araújo AS, Pinto JM, Neto AG, Viana CM, De Moraes Filho MO, Pereira ED. Comparison of the effects of pulmonary rehabilitation with chest physical therapy on the levels of fibrinogen and albumin in patients with lung cancer awaiting lung resection: a randomized clinical trial. BMC Pulmonary Medicine, v. 14, p. 121-127, 2014. [DOI]
127. Neto JB, da Silva CR, Neta MA, Campos RS, Siebra JT, Silva RA, Gaspar DM, Magalhães HI, de Moraes MO, Lobo MD, Grangeiro TB, Carvalho TS, Diogo EB, da Silva Júnior EN, Rodrigues FA, Cavalcanti BC, Júnior HV.Antifungal Activity of Naphthoquinoidal Compounds In Vitro against Fluconazole-Resistant Strains of Different Candida Species: A Special Emphasis on Mechanisms of Action on Candida tropicalis. PlosOne, v. 9, p. e93698, 2014.[DOI]
128. da Silva CR, de Andrade Neto JB, de Sousa Campos R, Figueiredo NS, Sampaio LS, Magalhães HI, Cavalcanti BC, Gaspar DM, de Andrade GM, Lima IS, de Barros Viana GS, de Moraes MO, Lobo MD, Grangeiro TB, Nobre Júnior HV.Synergistic Effect of the Flavonoid Catechin, Quercetin, or EpigallocatechinGallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole.AntimicrobialAgentsandChemotherapy, v. 58, p. 1468-1478, 2014.[DOI]
129. Ribeiro DG, Silva RP, Barboza DR, Lima-Júnior RC, Ribeiro RA.Clinical correlation between N-terminal Pro-B-Type Natriuretic paptide and angiographic coronary atherosclerosis.Clinics, v.69, p. 405-412, 2014.[DOI]
130. Skeff MA, BritoGAC, de Oliveira MG, Braga CM, Cavalcante MM, Baldim V, Holanda-Afonso RC, Silva-Boghossian CM, Colombo AP, Ribeiro RA, Moura-Neto V, Leitão RFC. S-nitrosoglutathione accelerates recovery from 5-fluorouracil-induced oral mucositis. PLoS ONE. v. 9, p. e113378, 2014.[DOI]
131. Silva RO, Lucetti LT, Wong DVT, Aragão KS, Junior EMA, Soares PMG, Barbosa ALR, Ribeiro RA, Souza MHLP, Medeiros JVR. Alendronate induces gastric damage by reducing nitric oxide synthase expression and NO/cGMP/KATP signaling pathway.Nitric Oxide. v. 40, p. 22-30, 2014.[DOI]
132. de Queiroz CAA, Fonseca SGC, Frota PB, Figueiredo TL, Aragão KS , Magalhães CEC, de Carvalho CBM, Lima ATM, Ribeiro RA, Guerrant RL, Moore SR, Oria RB. Zinc treatment ameliorates diarrhea and intestinal inflammation in undernourished rats.BMC Gastroenterology. v. 14, p.136, 2014.[DOI]
133. Costa MLV, Lima-Júnior RCP, Aragão KS, Medeiros RP, Marques-Neto RD, de Sá Grassi L, Leite LL, Nunes LG, de Mesquita Neto JWB, , de Castro Brito GA, de Souza MHLP, de Almeida PRC, Ribeiro RA. Chemotherapy-associated steatohepatitis induced by irinotecan: a novel animal model.Cancer Chemotherapy and Pharmacology. v. 74, Issue 4, p. 711-720, 2014.[DOI]
134. Justino PFC, Melo LFM, Nogueira AF, Costa JVG, Silva LMN, Santos CM, Mendes WO, Costa MR, Franco AX, Lima AA, Ribeiro RA, Souza MHLP, Soares PMG.Treatment with Saccharomyces boulardii reduces the inflammation and dysfunction of the gastrointestinal tract in 5-fluorouracil-induced intestinal mucositis in mice.British Journal of Nutrition.v. 111, p. 1611-1621, 2014.[DOI]
135. Goes P, Melo IM, Silva LMCM, Benevides NMB, Alencar NMN, Ribeiro RA, Lima V.Low-dose combination of alendronate and atorvastatin reduces ligature-induced alveolar bone loss in rats.Journal of Periodontal Research. v. 49, p. 45-54, 2014.[DOI]
136. Lima BB, Fonseca BF, Amado NG, Lima DM, Ribeiro RA, Abreu JG, de Castro Brito GA.Clostridium difficile toxin A attenuates Wnt/β-catenin signaling in intestinal epithelial cells.InfectionandImmunity. v.82, p. 2680-2687, 2014.[DOI]
137. Carvalho ACS, Sousa RB, Franco AX, Costa JVG, Neves LM, Ribeiro RA, Sutton R, CriddleDN, Soares PMG, de Souza MHLP. Protective effects of fucoidan, a P- and L-selectin inhibitor, in murine acute pancreatitis.Pancreas. v.43, p.82-87, 2014. [DOI]
138. Sampaio JPA, Cavalcante JR, Furtado FNN, Lima-Júnior RCP, Ribeiro RA, Almeida PRC.A handcrafted tissue microarray for a matrix arrangement of tissue samples.JournalofPharmacologicalandToxicologicalMethods. v.70, p.70-72, 2014. [DOI]
139. Batista JA, Dias EGN, Brito TV, Prudêncio RS, SilvaRO, Ribeiro RA, Souza MHLP, de Paula RCM, Feitosa JPA, Chaves LS, Melo MRS, Freitas ALP, Medeiros JVR, Barbosa ALR. Polysaccharide isolated from Agardhiellaramosissima: Chemical structure and anti-inflammation activity.Carbohydrate Polymers.v.99, p.59-67,2014.[DOI]
140. Wanderley CWS, Silva CMS, Wong DVT,Ximenes RM,Morelo DFC,Cosker F,Aragão KS,Fernandes C, Palheta-Júnior RC,Havt A, Brito GAC, Cunha FQ, Ribeiro RA, Lima-Júnior RCP.Bothropsjararacussu snake venom-induces a local inflammatory response in a prostanoid- and neutrophil-dependent manner. Toxicon. v. 90, p. 134-147,2014.[DOI]
141. Marinho RR, Matos RM, Santos JS, Ribeiro MAG, Ribeiro RA, Lima Jr. RCP, Albuquerque Jr. RLC, Thomazzi SM. Potential anti-inflammatory effect of low-level laser therapy on the experimental reflux laryngitis: A preliminary study.Lasers in Medical Science.v. 29, p. 239-243,2014. [DOI]
142. Vilar DDA, Vilar MSDA, Moura TFADLE, Raffin FN, Oliveira MRD, Franco CFDO, de Athayde-Filho PF, Diniz MFFM, Barbosa-Filho JM. Traditional Uses, Chemical Constituents, and Biological Activities of Bixaorellana L.: A Review. The Scientific World Journal, v. 2014, p. 1-11, 2014.[DOI]
143. Pita JC, Gomes IF, dos Santos SG, Tavares JF, da Silva MS, Diniz MFFM, Sobral MV. Matrix effect and optimization of LC-MSn determination of trachylobane-360 in mice blood.Journal of Pharmaceutical and Biomedical Analysis, v. 100 p. 262-270, 2014.[DOI]
144. Monteiro LS, Bastos KX, Barbosa-Filho JM, de Athayde-Filho PF, Diniz MFFM, Sobral MV. Medicinal Plants and Other Living Organisms with Antitumor Potential against Lung Cancer.Evidence-Based Complementary and Alternative Medicine, v. 2014, p. 1-15, 2014.[DOI]
145. Barreto E, Serra MF,Santos RV, Santos CEA, Hickmann J,Cotias AC, Pão CRR,Trindade SG, Schimidt V, Giacomelli C, Carvalho VF, e Silva PM, CordeiroRSB, Martins MA. Local administration of gold nanoparticles prevents pivotal pathological changes in murine models of atopic asthma. Journal of Biomedical Nanotechnology.v.10, p. 1-13, 2014.[DOI]
146. Fernandes PD, Zardo RS, Figueiredo GSM, Silva BV, Pinto AC. Anti-inflammatory properties of convolutamydine A and two structural analogues. Life Sciences, v. 116, p. 16-24, 2014.[DOI]
Academic Production
FINISHED MASTER DISSERTATIONS IN 2014
1. Luiz Claudio T. de Souza. Micro
and small software companies in the realm of current national
policies for supporting innovation and intellectual property case
study: Rede Rio TI Serviços. 2014. Dissertation (Professional
Master’s Degree in Industrial Property and Innovation) – National
Institute of Industrial Property. Advisor: Adelaide Maria de Souza
Antunes.
2. Alexandre
Pinhel Soares. Nanotechnology in the Electrical Sector: A
Prospective Study. 2014. Dissertation (Professional Master’s Degree
in Industrial Property and Innovation) – National Institute of
Industrial Property. Advisor:
Adelaide Maria de Souza Antunes.
3. David Oliveira Pinheiro Junior. Transfer of technology between ICT and pharmaceutical
company: Emphasis on the valuation of intangible actives. 2014.
Dissertation (Professional Master’s Degree in Industrial Property
and Innovation) – National Institute of Industrial Property.
Advisor: Adelaide Maria de Souza
Antunes.
4. Jenny Zorayda Garavito Najas. Study of cyclotides in Violaceae from the Tropical Forest in
the state of Rio de Janeiro: isolation and structural
characterization. 2014. Dissertation (Master’s Degree in Chemistry)
– Federal University of Rio de Janeiro, Coordination for the
Improvement of Higher Education Personnel. Advisor: Angelo da Cunha
Pinto.
5. Thaise da
Silva Martins. Studies aimed at the discovery of ALDH-2 inhibitors
useful in the treatment of cocaine dependency. 2014. Dissertation
(Master’s Degree in Chemistry) – Federal University of Rio de
Janeiro, Coordination for the Improvement of Higher Education
Personnel. Advisor: Carlos
Alberto Manssour Fraga.
6. Fernando Rodrigues Mathias da Silva Seixas.
Studies of Molecular Modelling and Structural
Planning of new Candidates Inhibitors of Adenosine Deaminase. 2014.
Dissertation (Master’s Degree in Biological Science (Pharmacology
and Medicinal Chemistry)) – Federal University of Rio de Janeiro.
Advisor: Carlos Alberto Manssour Fraga.
7. Sarah
Teixeira Silva. In vitro
study of biomarker potential of nephrotoxicity
through genic expression using gentamicin. 2014. Dissertation
(Master’s Degree in Biopharmaceutical Innovation) – Federal
University of Minas Gerais, Coordination for the Improvement of
Higher Education Personnel. Advisor: Carlos Alberto Tagliati.
8. Sheisi Fonseca Leite da Silva Rocha. Development of an Empirical Model of Prediction of Activity
of Urease Inhibitors using the Semi-Empirical Method PM6. 2014.
Dissertation (Master’s Degree in Chemistry) – Federal Rural
University of Rio de Janeiro, Coordination for the Improvement of
Higher Education Personnel. Advisor: Carlos Mauricio Rabello de Sant'Anna.
9. Marianna Ramos dos Anjos. Multiresidue method for analysis of aflatoxin M1,
avermectins, organophosphate agrotoxins and milbemycin in milk
through ultraefficcient chromatography coupled with sequential mass
spectrometry. 2014. Dissertation (Master’s Degree in Food Science)
– Federal University of Rio de Janeiro. Advisor: Francisco Radler
de Aquino Neto.
10. Daniella
Moreira Leal. Pharmacological evaluation of
Pyrazole[3,4-B]Pyrrolo[3,4-D]Pyridine Derivate in animal model of
acute chronic pain. 2014. Dissertation (Master’s Degree in
Biological Sciences (Pharmacology and Medicinal Chemistry)) –
Federal University of Rio de Janeiro, Coordination for the
Improvement of Higher Education Personnel. Advisor: Gisele
Zapata-Sudo.
11. Carla
Moreira Leal. Pharmacological evaluation of new N-acylhydrazone heteroaromatic
derivate for the treatment of arterial hypertension and pulmonary
arterial hypertension. 2014. Dissertation (Master’s Degree in
Biological Sciences (Pharmacology and Medicinal Chemistry)) –
Federal University of Rio de Janeiro. Advisor: Gisele
Zapata-Sudo.
12. Paulo
Roberto Teixeira Werdt. Prediction of protein structures using RMN
restrictions and a Coarse Grained model. 2014. Dissertation
(Master’s Degree in Computational Modelling) – National Laboratory
of Scientific Computation, Coordination for the Improvement of
Higher Education Personnel. Advisor: Laurent Emmanuel
Dardenne.
13. Karina
Baptista dos Santos. Prediction of protein structure using dihedral
angle restrictions. 2014. Dissertation (Master’s Degree in
Computational Modelling) – National Laboratory of Scientific
Computation, Coordination for the Improvement of Higher Education
Personnel. Advisor: Laurent Emmanuel Dardenne.
14. Paula
Kishi Kuroishi. Total synthesis of (-)-Cryptocaryol A. 2014.
Dissertation (Master’s Degree in Chemistry) – State University of
Campinas, Coordination for the Improvement of Higher Education
Personnel. Advisor: Luiz Carlos Dias.
15. Maria
Alice Pimentel Falcao. Evaluation of the anti-inflammatory and
antinociceptive action mechanism of Caulerpa kempfii. 2014. Dissertation
(Master’s Degree in Health Sciences) – Federal University of
Alagoas, Coordination for Improvement of Higher Education
Personnel. Advisor: Magna Suzana Alexandre Moreira.
16. Francisco
Stefanio Barreto. Study of cytotoxic activity of compounds obtained
from the acetonic extract from the leaves of Annona muricataL. through bioacute
fractioning. 2014. Dissertation (Master’s Degree in Pharmacology) –
Federal University of Ceara, National Council of Scientific and
Technological Development. Advisor: Manoel Odorico de Moraes Filho.
17. Cristiano Walter Moraes Rola Junior.
Profile of patients sent to admission at ICU
through the Central of Regulation of beds in Fortaleza. 2014.
Dissertation (Master’s Degree in Pharmacology) – Federal University
of Ceara. Advisor: Manoel Odorico de Moraes Filho.
18. Italo
Savio Mendes Rodrigues. Biomonitoring of leather workers exposed at
work to chemical mixtures containing chrome III through measuring
of chrome in urine and from complete assay in Teresina-PI. 2014.
Dissertation (Master’s Degree in Graduate Program in Physiology and
Pharmacology) – Federal University of Ceara. Advisor: Manoel
Odorico de Moraes Filho.
19. Amanda da
Costa Cotias. Effect of bupivacaine metabolite, pipecolic xylidine,
in experimental asthma refractory to glucocorticoids. 2014.
Dissertation (Master in Human and Experimental Biology) – State
University of Rio de Janeiro, Carlos Chagas Filho Foundation of
Support to Research in the State of RJ. Advisor: Marco Aurelio
Martins.
20. Camila Ribeiro Rodrigues de Pao. Development and validation of an experimental model of asthma
refractory to glucocorticoids. 2014. Dissertation (Master’s Degree
in Biological Science (Pharmacology and Medicinal Chemistry)) –
Federal University of Rio de Janeiro. Advisor: Marco Aurelio
Martins.
21. Thais
Biondino Sardella. Isatin, n-methyl-isatin and
n-methyl-3-(2-oxopropil)-3-hydroxy-2-oxindol: antinociceptive
profile and action mechanism. 2014. Dissertation (Master’s Degree
in Biological Sciences (Pharmacology and Medicinal Chemistry)) –
Federal University of Rio de Janeiro, Coordination for the
Improvement of Higher Education People. Advisor: Patricia Dias
Fernandes.
22. Natalia de
Morais Sales. Evaluation of LASSBio-1524 and three new analogs in a
new acute inflammation model. 2014. Dissertation (Master’s Degree
in Biological Sciences (Pharmacology and Medicinal Chemistry)) –
Federal University of Rio de Janeiro, Coordination for the
Improvement of Higher Education Personnel. Advisor: Patricia Dias
Fernandes.
23. Ananssa Maira dos Santos Silva. Effect of incorporation of dantrolene and azumolene in
β-cyclodextrin in the regulation of muscular contractibility.
2014. Dissertation (Master’s Degree in Biological Sciences
(Pharmacology and Medicinal Chemistry)) – Federal University of Rio
de Janeiro, National Council for Scientific and Technological
Development. Advisor: Roberto
Takashi Sudo.
24. Rachel do Amaral Ribeiro Araujo Vieiralves.
New alfa-2 adrenergic agonist with analgesic
efficacy on animal chronic pain model. 2014. Dissertation (Master’s
Degree in Biological Sciences (Pharmacology and Medicinal
Chemistry)) – Federal University of Rio de Janeiro, Advisor:
Roberto Takashi Sudo.
25. Victor Jose Goncalves de Moura. New N-acylhydrazone derivate, LASSBio-1359, drug candidate for the
treatment of erectile dysfunction. 2014. Dissertation (Master’s
Degree in Biological Sciences (Pharmacology and Medicinal
Chemistry)) – Federal University of Rio de Janeiro. Advisor:
Roberto Takashi Sudo.
26. Renata
Machado Brandao Costa. Impact of extracellular matrix derived from
melanoma on the angiogenic profile of endothelial cells:
Involvement of Integrin dependent pathways. 2014. Dissertation
(Master’s Degree in Biosciences) – Federal University of Rio de
Janeiro, Coordination for the Improvement of Higher Education
Personnel. Advisor: Thereza Christina Barja Fidalgo.
FINISHED DOCTORAL THESES IN 2014
1. Cristina
d'Urso de Souza Mendes. Future vision for the production of
antibiotics: research, development, and innovation trends. 2014.
Thesis (Doctorate in Technology of Chemical and Biochemical
Processes) – School of Chemistry – UFRJ. Advisor: Adelaide Maria de Souza Antunes.
2. Flavia
Maria Lins Mendes. Methodology for the identification of key
intermediates of synthetic active principles. Case Study:
Antiretrovirals for the treatment of AIDS. 2014. Thesis (Doctorate
in Technology of Chemical and Biochemical Processes) – School of
Chemistry – UFRJ. Advisor:
Adelaide Maria de Souza Antunes.
3. Viviane
Masseran Antunes Parreiras. Proposal of trend observatory in a
business R&D center – case of nanotechnology at CENPES. 2014.
Thesis (Doctorate in Technology of Chemical and Biochemical
Processes – School of Chemistry - UFRJ. Advisor: Adelaide Maria de Souza Antunes.
4. Sabrina Dias de Oliveira. Analysis of the Production of succinic acid from renewable
sources: perspectives and challenges. 2014. Thesis (Doctorate in
Technology of Chemical and Biochemical Processes) – School of
Chemistry – UFRJ. Advisor:
Adelaide Maria de Souza Antunes.
5. Fabio Teixeira da Silva. Ficus genus.
Studies of historical and chemical aspects. 2014.
Thesis. (Doctorate in Chemistry). Federal University of Rio de
Janeiro, Coordination for the Improvement of Higher Education
Personnel. Advisor: Angelo da Cunha Pinto.
6. Daniel Rosa
da Silva. Development of Empirical Models of Prediction of Activity
of Acetylcholinesterase Enzyme of Torpedo
californica and Aedes aegyptiusing the
Semi-Empirical Method. 2014. Thesis (Doctorate in Chemistry) –
Federal Rural University of Rio de Janeiro, Coordination for the
Improvement of Higher Education Personnel. Advisor: Carlos Mauricio
Rabello de Sant'Anna.
7. Lana
Gabriela. Mapping and legal approaches of bioprospecting networks
in Brazil. 2014. Thesis (Doctorate in Biotechnology - RENORBIO) –
State University of Ceara, National Council for Scientific and
Technological Development. Advisor: Claudia do Ó Pessoa.
8. Jose Rubens
Costa Lima. Monitoring of venous hydration in the hemorrhagic fever
in dengue and other pathologies. 2014. Thesis (Doctorate in
Biotechnology - RENORBIO) – State University of Ceara. Advisor:
Claudia do Ó Pessoa.
9. Paula
Giselle Czaikoski. Role of NET in the evolution of sepsis. 2014.
Thesis (Doctorate in Pharmacology) – Faculty of Medicine of
Ribeirao Preto - USP, Foundation for the Support of Research in the
State of Sao Paulo. Advisor: Fernando de Queiroz Cunha.
10. Gabriela
Trentin Scortegagna. Physiopathological mechanisms involved in the
susceptibility of NOD (non-obese diabetic) mice to sepsis, role of
NETs (neutrophil extracellular traps), AGP (alfa-1 acid
glycoprotein), and Histamine. 2014. Thesis (Doctorate in Basic and
Clinical Immunology) – Faculty of Medicine of Ribeirao Preto – USP,
Foundation for the Support of Research in the State of Sao Paulo.
Advisor: Fernando de Queiroz Cunha.
11. Jaqueline
Raymondi Silva. Neuro-immune interactions involved in the genesis
and maintenance of nociceptive herpetic hypersensitivity and
post-herpetic. 2014. Thesis (Doctorate in Immunology) – Faculty of
Medicine of Ribeirao Preto – USP, Foundation for the Support to
Research in the State of Sao Paulo. Advisor: Fernando de Queiroz
Cunha
12. Guilherme
Carneiro Montes. Investigation of the activity of
N-methyl-Acylhydrazone
derivates (LASSBio-1359 and LASSBio-1289) in central nervous
system. 2013. Thesis (Doctorate in Biological Sciences
(Pharmacology and Medicinal Chemistry)) – Federal University of Rio
de Janeiro. Advisor: Gisele Zapata-Sudo.
13. Danilo
Pereira de Sant'Ana. Synthesis of the C1-C9 fragment of
(-)-dictyostatin and studies aimed at the total synthesis of
(+)-tautomycetin. 2014. Thesis (Doctorate in Chemistry) – State
University of Campinas, Foundation for Support of Research in the
State of Sao Paulo. Advisor: Luiz Carlos Dias.
14. Yolanda
Karla Cupertino da Silva. Evaluation of immune modulating activity
of new N-acylhydrazone (NAH) pyrazine derivates: a proposal for the
discovery of an antitumoral/immunosuppresor drug. 2014. Thesis
(Doctorate in RENORBIO) – Northeast Network of Biotechnology,
Coordination for the Improvement of Higher Education Personnel.
Advisor: Magna Suzana Alexandre Moreira.
15. Flavia
Castelo Batista Magalhaes. From legal access and biological
material to patenting of biotechnological product: Brazilian
dimensions and challenges. 2014. Thesis (Doctorate in Biotechnology
– RENORBIO) – State University of Ceara. Advisor: Manoel Odorico de
Moraes Filho.
16. Francisco
Jose Lopes Cajado. Use of liquid extract of the cactus pear,
(Opunta ficus indica), as a diluting agent and criopreservative of the semen of
fish cultivated in the Brazilian Northeast. 2014. Thesis (Doctorate
in Biotechnology - RENORBIO) – State University of Ceara. Advisor:
Manoel Odorico de Moraes Filho.
17. Diana
Dalzy Viveiros. Effect of the N-acylhydrazone LASSBio-897 derivate
on the inflammatory pulmonary response in experimental asthma and
silicosis. 2014. Thesis (Doctorate in Cellular and Molecular
Biology) – Oswaldo Cruz Foundation, Coordination for the
Improvement of Higher Level Personnel. Advisor: Marco Aurelio
Martins.
18. Suzana
Vanessa Soares Cardoso. Triage and pharmacological evaluation of
new PDE4 inhibitors, of N-methylacylhydrazones, for asthma
control. 2014. Thesis (Doctorate in Cellular and Molecular Biology)
– Oswaldo Cruz Foundation, Oswaldo Cruz Institute. Advisor: Marco
Aurelio Martins.
19. Monica
Lorena Dias Meireles. Clinical pharmacological Stage I and II
assays with synthetic compound 5,7-Diacetox-4-Arylcromane,
derivates of constituents of the Coutarea
hexandra species in the treatment of
herpes simplex. 2014. Thesis (Doctorate in Natural and Synthetic
Bioactive Products) – Federal University of Paraiba.
Advisor: Margareth de Fatima Formiga
Melo Diniz.
20. Daiene
Martins Lunguinho. Studies of Antitumoral and Toxicological Effects
of the Essential Oil from the Leaves of Xylopia frutescens Aubl.
(ANNONACEAE). 2014. Thesis (Doctorate in Natural and Synthetic
Bioactive Products) – Federal University of Paraiba.
Advisor: Margareth de Fátima Formiga
Melo Diniz.
21. Heraldo Arcela de Carvalho Rocha. Clinical Assay with the Extract from the Roots of
Panax Ginseng C. A. Meyer
in the Treatment of Irritable Bowel Syndrome. 2014. Thesis
(Doctorate in Natural And Synthetic Bioactive Products) – Federal
University of Paraiba. Advisor: Margareth de Fatima Formiga Melo
Diniz.
22. Cynthia
Samary. Impact of transpulmonary pressures generated by the
combination of different volumes and positive pressures at the end
of expiration in an acute respiratory distress model. 2014. Thesis
(Doctorate in Biological Sciences – Physiology) – Federal
University of Rio de Janeiro, Coordination for the Improvement of
Higher Education Personnel. Advisor: Patricia Rieken Macedo
Rocco.
23. Johnatas
Dutra Silva. Effects of different mesenchymal cells in Acute
Pulmonary Lesion model. 2014. Thesis (Doctorate in Biological
Sciences – Physiology) – Federal University of Rio de Janeiro,
Coordination for the Improvement of Higher Education Personnel.
Advisor: Patricia Rieken Macedo Rocco.
24. Milene
Borsoi. Repeated forced swimming as a paradigm for the study of
cognitive damage and synaptic plasticity changes related to
neuropsychiatric illnesses. 2014. Thesis (Doctorate in Biological
Sciences (Neuroscience)) – Federal University of Rio Grande do Sul,
Coordination for the Improvement of Higher Education Personnel.
Advisor: Stela Maris Kuze Rates.
25. Liz
Girardi Müller. Study of the involvement of different biological
targets in the mechanism of antidepressive action of a fraction
enriched in dienic valepotriates obtained from Valeriana
glechomifolia Meyer (Valerianaceae) of Valeriana glechomifolia
Meyer (Valerianaceae). 2014. Thesis (Doctorate in Pharmaceutical
Sciences) – Federal University of Rio Grande do Sul, Coordination
for the Improvement of Higher Education Personnel. Advisor: Stela
Maris Kuze Rates.
26. Antonio de
Moraes Izquierdo. Expression of molecular markers of the process of
radicular reabsorption induced by successive cycles of orthodontic
movement. 2014. Thesis (Doctorate in Dentistry) – Federal
University of Rio de Janeiro, Coordination for the Improvement of
Higher Education Personnel. Advisor: Thereza Christina Barja
Fidalgo.
INCT-INOFAR Scholarships
FIOCRUZ/RJ
1) Bianca
Torres Ciambarella CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: July
2013 to January 2014
Project:
“Studies of potential cellular targets and
action mode of LASSBio-897 compound in control of experimental
silicosis.”
Advisor: Prof. Dr. Patricia Machado Rodrigues e Silva
Martins
2) Julio
Beltrame Daleprane CV-Lattes
CNPq
Technological Development Scholarship – DTI-1
Time: December
2011 to February 2012
Project: “Study of the potential
anti-inflammatory effect of compound LASSBio 897, in models of
silicosis and asthma.”
Advisor: Prof. Dr. Marco Aurelio Martins
3) Vinicius de Frias Carvalho CV-Lattes
CAPES
Post-doctoral Scholarship
Time: March
2010 to February 2012
Project:
“Study of pharmacological interaction of
LASSBio-897 and LASSBio-294 with adenosine receptors in living
cells.”
Advisor: Prof. Dr. Marco Aurélio Martins
UNIFAL
4) Andre
Victor Pereira CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time: June
2012 to May 2013, September 2013 to June 2014 and August 2014 to
December 2014
Project:
“Technological foresight of intermediaries
and synthetic chemical entities of interest in the scope of the
INCT-INOFAR.”
Advisor: Prof. Dr. Marcia Paranho Veloso
UNICAMP
5) Adriano
Siqueira Vieira CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: August
2009 to June 2011
CNPq
Technological Development Scholarship – DTI-1
Time: July
2011 to March 2012
Project:
“Atorvastatin synthesis”
Advisor: Prof. Dr. Luiz Carlos Dias
Institute of
Chemistry
6) Elsa Moreno de Viguri CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: April
2013 to March 2014
Project:
“New quinic acid derivatives as
Trypanosoma cruzi trans-sialidase inhibitors”
Advisor: Prof. Dr. Luiz Carlos Dias
Institute of
Chemistry
7) Javier
Ceras Aresse CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: April
2013 to March 2014
Project:
“Synthesis of Valsartan”
Advisor: Prof. Dr. Luiz Carlos Dias
Institute of
Chemistry
8) Leila de Souza Conegero CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: July
2010 to January 2011
Project:
“Fluoxetine synthesis”
Advisor: Prof. Dr. Luiz Carlos Dias
Institute of
Chemistry
9) Maitia
Labora Poggi CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: March
2014 to October 2014
Project:
“Development of new anti-Chagas
trans-sialidase inhibitors”
Advisor: Prof. Dr. Luiz Carlos Dias
Institute of
Chemistry
UFC
10) Bruno
Coelho Cavalcanti CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: May 2010
to December 2010
Project: “In
vitroevaluation of cytotoxic, genotoxic
and mutagenic potential of samples provided by
INCT-INOFAR.”
Advisor: Prof. Dr. Leticia Veras Costa Lotufo
Unity of Clinical Pharmacology
UFG
11) Ana Maria Calado Dos Santos CV-Lattes
CNPq Technical
Support Scholarship – AT NM
Time: July
2010 to June 2011
Project: “In
silicoprediction and in vitro production
of pharmaceutical prototype candidates through bioconversion of
human metabolites”
Advisor: Prof. Dr. Valeria de Oliveira
Faculty of
Pharmacy
12) Geovana
Barbara Ferreira Mendes CV-Lattes
CNPq Technical
Support Scholarship – AT NM
Time: March
2013 to January 2014
Project: “In
silicoprediction and in vitroproduction of pharmaceutical
prototype candidates through bioconversion of human
metabolites”
Advisor: Prof. Dr. Valeria de Oliveira
Faculty of
Pharmacy
13) Sarah da Silva Nunes CV-Lattes
CNPq Technical
Support Scholarship – AT NM
Time: July
2011 to December 2011, February 2012 to July 2012 and September
2012 to February 2013
Project: “In
silicoprediction and in vitroproduction of pharmaceutical
prototype candidates through bioconversion of human
metabolites”
Advisor: Prof. Dr. Valeria de Oliveira
Faculty of
Pharmacy
UFMG
14) Carolina
Neris Cardoso CV-Lattes
CNPq
Technological Initiation – ITI A
Time:
September 2011 to January 2012
Project: “Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof.
Dr. Carlos Alberto Tagliatti
Faculty of
Pharmacy
15) Carolina
Maldonado Galassi CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time: October
2009 to March 2013
Project: “Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of
Pharmacy
16) Gabrielle
Luck de Araujo CV
Lattes
CNPq Junior
Post-Doctorate Scholarship – PDJ
Time: July to
December 2011
Project:
“Semicarbazone Benzaldehyde (BS):
toxicological aspects”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of
Pharmacy
17) Isabella
Pires Ferreira CV
Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: June to
November 2014
Project:
“Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof. Dr. Heloisa de Oliveira Beraldo
Institute of Exact Sciences
18) Manuela de Lima Toccafondo Vieira CV Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: May to
October 2013
Project:
“Modelling and PBPK Simulation of
LASSBIO-596 Compound”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of
Pharmacy
19) Marcus Vinicius dos Santos CV-Lattes
CNPq
Technological Initiation – ITI A
October 2009
to March 2010
Project: “Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of
Pharmacy
20) Nathalia
Freitas Emiliano CV-Lattes
CNPq
Technological Initiation – ITI A
Time:
September 2011 to January 2012
Project: “Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof. Dr. Carlos Alberto Tagliatti
Faculty of
Pharmacy
21) Samira de Sa e Souza CV-Lattes
CNPq
Technological Initiation – ITI A
Time:
September 2011 to January 2012
Project: “Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof.
Dr. Carlos Alberto Tagliatti
Faculty of
Pharmacy
22) Wallace
Carvalho Ferreira CV-Lattes
CNPq Technical
Support Grant– AT NM
Time: August
2009 to January 2010
Project: “Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof. Dr. Marcio de Matos Coelho
Faculty of
Pharmacy
UFRGS
23) Moacir
Kaiser CV-Lattes
CNPq
Technological Development Grant– DTI-3
Time: July
2009 to March 2010
Project: “Evaluation of
pharmakinetic profile of LASSBio-468.”
Advisor: Prof.
Stella Maris Kuze Rates
UFRJ
24) Allan Kardec Nogueira de Alencar CV-Lattes
CNPq Technical
Support Grant– AT NM
Time: April to
August 2010
Project:
“Development of new substances for the
reduction of ventricular dysfunction, caused by arterial and
pulmonary hypertension.”
Advisor: Prof.
Roberto Takashi Sudo
Institute of
Biological Sciences (ICB)
25) Alan Rodrigues de Sousa CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time: February
2012 to June 2014
CNPq
Technological Support Scholarship – AT NM
Time: August
to 2012 to August 2013
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
26) Alexandra Basilio Lopes CV-Lattes
CNPq
Technological Development Grant– DTI-3
Time: June to
September 2010
Project: “Synthesis and evaluation
of antinociceptive and anti-inflammatory activities of
phenyl-pyridine-N-acylhydrazone compounds planned from imidazo [1,2-a]
pyridine-N-acylhydrazone derivatives.”
Advisor: Prof. Eliezer J. Barreiro
LASSBio
27) Ana Carla Dos Santos CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time: July
2009 to June 2010
CNPq
Technological Development Scholarship – DTI-2
Time: July to
2010 to June 2011
CNPq
Technological Development Scholarship – DTI-1
Time: July
2011 to March 2012
CNPq Technical
Support Scholarship – AT NS
Time: April
2012 to August 2012
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
28) Ana Cristina da Mata Silva CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time: April
2012 to May 2013
CNPq
Technological Development Scholarship – DTI-2
Time: June
2013 to August 2014
CNPq
Technological Development Scholarship – DTI-1
Time:
September 2014 to March 2015
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
29) Ana Gabriela de Almeida Silva CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time: March
2013 to August 2013
Project:
“Implementation and validation of
pre-clinical trial model for the evaluation of the teratogenic
effect of bioactive substances: evaluation of the LASSBio 468 and
LASSBio 596 prototypes”
Advisor: Prof. Dr. Aloa Machado de Souza
LASSBio
30) Arthur
Eugen Kümmerle CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
September 2009
to March 2010
Project: “Study of the Inclusion of
LASSBio-579 in cyclodextrin.”
Advisor: Prof. Eliezer J. Barreiro
LASSBio
31) Arthur Henrique Freitas do Prado CV-Lattes
CNPq Technical
Support Scholarship – AT NS
Time: May 2011
to February 2012
Project:
“Scientific awareness and health education
at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
32) Barbara Assis Novak CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time:
September 2012 to February 2013
Project:
“Implementation and validation of
pre-clinical trial model for the evaluation of the teratogenic
effect of bioactive substances: evaluation of the LASSBio 468 and
LASSBio 596 prototypes”
Advisor: Prof. Dr. Aloa Machado de Souza
LASSBio
33) Carlos Eduardo da Silva Monteiro CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time: May 2010
to February 2011
Project:
“Multitarget activation: strategy for
symptomatic treatment of neuropathic pain”
Advisor: Prof.
Roberto Takashi Sudo
Institute of
Biological Sciences (ICB)
34) Clemilson
Berto Junior CV-Lattes
CAPES Master
Scholarship
Time: October
2011 to March 2012
Project:
“Evaluation of teratogenic potential of
LASSBio 596 and LASSBio 468 prototypes, antiasthma pharmaceutical
candidates”
Advisor: Prof. Dr. Aloa Machado
LASSBio
35) Daniel Nascimento do Amaral CV-Lattes
CAPES Master
Scholarship
Time: March
2010 to February 2012
Project: “Design, synthesis and pharmacological evaluation of new
antitumor ß –tubulin inhibitor prototypes”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
36) Douglas Rodrigues Outeiro de Oliveira CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time:
September 2013 to November 2014
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
37) Edna Maria de Oliveira Ferreira CV-Lattes
CNPq
Technological Development Scholarship – DTI-1
Time:
September 2014 to November 2015
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
38) Fabricio Maia da Silva Salvador CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time: October
2012 to July 2013
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
39) Fanny Nascimento Costa CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: July
2013 to December 2013
Project: “The use of polycrystalline
X-ray diffraction in the structural determination of new drug
candidate N-acylhydrazone derivatives.”
Advisor: Prof. Eliezer J. Barreiro
LASSBio
40) Givanildo Santos da Silva CV-Lattes
CAPES Doctoral
Grant
October 2009
to August 2010
Project: “Studies for the discovery
of new anti-influenza, neuraminidase inhibitor
prototypes.”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
41) Hannah Carolina Tavares Domingos CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time:
September 2011 to February 2012
Project: “Qnint”
Advisor: Prof.
Dr. Claudia Rezende
Institute of
Chemistry
42) Jean
Marcell Marcelino Pena CV-Lattes
CNPq Technical
Support Grant– AT NM
From November
2013 to June 2014 and September to December 2014
Project:
“Development of a new synthetic route for
preparation of generic drugs clozapine and quetiapine”
Advisor: Prof. Dr. Angelo da Cunha Pinto
Institute of
Chemistry
43) Jessica Silva dos Santos CV-Lattes
CNPq Technical
Support Grant– AT NM
From October
to December 2010
Project:
“Scientific awareness and health education
at INCT-INOFAR”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
44) Juliana Fatima Vilacha Madeira Rodrigues dos
Santos CV Lattes
CNPq Technical
Support Scholarship – IC
Time: March
2012 to Mar 2013
Project:
“Planning, synthesis, and pharmacological
evaluation of 1,2,3,4-tetrahydroacridine derivates,
acetylcholinesterase inhibitor prototypes.”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
45) Leandro Louback da Silva CV-Lattes
CAPES Doctoral
Grant
Time: October
2009 to August 2010
Project: “Study of the effects of
different N-acylhydrazone derivatives on the cell-to-cell interaction
mechanisms and inflammatory mediators that are part of the
atherosclerotic process.”
Advisor: Prof. Dr. Ana Luisa Palhares de Miranda
LASSBio
46) Leonardo Ferreira de Oliveira CV-Lattes
CNPq Technical
Support Grant– AT NM
From December
2014 to December 2015
Project:
“Scientific awareness and health education
at INCT-INOFAR”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
47) Lethycia Machado Tannuri CV-Lattes
CNPq
Technological Development Scholarship – DTI-1
Time: November
2014 to November 2015
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof.
Dr. Eliezer J. Barreiro
LASSBio
48) Lidilhone
Hamerski Carbonezi CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: August
2010 to January 2011
Project:
“Sunitinib synthesis”
Advisor: Prof. Dr. Angelo da Cunha Pinto
Institute of
Chemistry (IQ)
49) Lucia
Beatriz Torres CV-Lattes
CNPq
Technological Development Scholarship – DTI-2
Time: October
2010 to September 2011
CNPq
Technological Development Scholarship – DTI-1
Time: October
2011 to July 2012 and July 2013 to February 2014
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
50) Luciana Almeida Piovesan CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: February
2009 to August 2009
Project:
“Design, Synthesis and Pharmacological
Evaluation of Novel Anti-Cancer Drug-Candidate
Prototypes”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
51) Luciano da Silva Santos CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time: August
2011
CNPq Technical
Support Scholarship – AT NS
Time:
September 2011 to February 2012
Project:
“Synthesis and pharmacological activity of
new ferrocene-N-acylhydrazone derivates”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
52) Luis Eduardo Reina Gamba CV-Lattes
CAPES Doctoral
Grant
Time: March
2014 to December 2014
Project: “Design and Synthesis of
New Hypoglycemiant DPP4 Inhibitors.”
Advisor: Prof. Dr. Ana Luisa Palhares de Miranda
LASSBio
53) Maria de Fatima do Nascimento Alfredo CV-Lattes
CNPq Technical
Support Scholarship – AT NS
Time: January
2012 to September 2013
Project:
“Scientific awareness and health education
at INCT-INOFAR”
Advisor: Prof.
Dr. Eliezer J. Barreiro
LASSBio
54) Mariana
Trad Rosner da Motta CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time: August
2011 to June 2012
Project:
“In vitro metabolism of new leishmanicidal
and tripanomicidal pharmaceutical prototypes”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
55) Marlon
Daniel Lima Tonin CV-Lattes
CNPq Technical
Support Scholarship – DTI-3
Time: April to
July 2012
Project:
“Novel 5-aryl-2-furfuryl-N-acylhydrazone
derivatives with potent anti-inflammatory and analgesic activity:
LASSBio-1609 and LASSBio-1636”
Advisor: Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio
56) Nailton Monteiro Nascimento Junior CV-Lattes
CAPES Exchange
Doctorate Scholarship (Dsw)
Time: March to
August 2012
Project:
“Virtual screening synthesis and
pharmacological evaluation of GPCRs ligands"
Advisor: Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio
57) Natalia Lacerda Alencar Peixoto CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time: December
2014 to December 2015
Project:
“Synthesis of cyclodextrin complexes of
LASSBio-596 salts”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
58) Natalia Medeiros de Lima CV-Lattes
CNPq Technical
Support Scholarship – AT NS
Time: August
2010 to July 2011
CNPq Technical
Support Scholarship – DTI-2
Time: July
2013 to December 2015
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
59) Pedro Gabriel Dias Lobato Pereira CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time: August
to October 2011 and January 2012 to June 2012
Project:
“Synthesis of cyclodextrin complexes of
LASSBio-596 salts”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
60) Priscila de Paula Cabral CV-Lattes
CNPq
Technological Development Scholarship – DTI-3
Time: May 2012
to June 2012
Project: “Scientific awareness and
health education at INCT-INOFAR”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
61) Raquel de Oliveira Lopes
CV-Lattes
CNPq Technical
Support Scholarship – DTI-3
Time: October
2010 to December 2010
Project: “Metabolic studies of
LASSBio-596”
Advisor: Prof.
Dr. Eliezer J. Barreiro
LASSBio
62) Roberta
Tesch CV-Lattes
CNPq Technical
Support Scholarship – AT NS
Time: June
2010 to July 2010
CAPES Master
Scholarship
Time: March to
April 2011
Project:
“Studies of molecular modeling and
structural planning of new ligands to adenosine
receptors”
Advisor: Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio
63) Rodolfo do Couto Maia CV-Lattes
CAPES Exchange
Doctorate Scholarship (Dsw)
Time: February
to July 2011
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: April
2012 to May 2012
Project:
“Synthesis and evaluation of antitumor
activity of a new family of pyrazole-pyridone
family"
Advisor:
Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio
64) Sabrina Teixeira Martinez CV-Lattes
CNPq Technical
Support Scholarship – DTI-1
Time:
September 2013 to January 2014
Project: “Bibliographical review of
methodologies of synthesis of clozapine and quetiapine”
Advisor: Prof. Dr. Angelo da Cunha Pinto
Institute of
Chemistry
65) Tais Rubia dos Santos CV-Lattes
CNPq
Scientific Initiation Scholarship - IC
Time:
September to November 2011, January to June 2012 and September 2012
to February 2013
Project: “Planning, synthesis and
pharmacological evaluation of new leflunomide
analogs”
Advisor: Prof. Dr. Lidia Moreira Lima
LASSBio
66) Thais Emanoelle Tavares Pompeu CV-Lattes
CAPES Doctoral
Grant
Time: June
2012 to August 2012
Project: “New approaches for the in
vitro studies of new N-phenylpiperazines candidates to new atypical
antipsychotics.”
Advisor: Prof.
Dr. François Germain Noël
LASSBio
67) Thiago
Stevanatto Sampaio CV-Lattes
CNPq Technical
Support Grant– AT NM
April 2009 to
March 2010
Project:
“Design, synthesis and evaluation of
cytotoxic properties of new TK inhibitor pharmaceutical candidate
prototypes.”
Advisor: Prof. Dr. Eliezer J. Barreiro
LASSBio
UNIVASF- PE
68) Juliane
Cabral Silva CV-Lattes
CNPq Technical
Support Scholarship – DTI-1
Time:
September 2014 to December 2014
Project: “Bibliographical review of
methodologies of synthesis of clozapine and quetiapine”
Advisor: Prof. Dr. Jackson Roberto da Silva
Guedes
Center for
Study and Research of Medicinal Plants
USP- RIBEIRAO PRETO
69) Ana Katia dos Santos CV-Lattes
CNPq Technical
Support Scholarship – AT NM
Time: January
to June 2012, August 2012 to June 2014 and August to December
2014
Project: “Semicarbazone Benzaldehyde (BS)”
Advisor: Prof. Dr. Fernando de Queiroz Cunha
Faculty of
Medicine of Ribeirao Preto
70) Danilo
Roman Campos CV-Lattes
CNPq Junior
Post-Doctorate Scholarship - PDJ
Time: February
2013 to December 2013
Project:
“Determination of the selectivity of
compounds LASSBio-1609 and LASSBio-1825 on the blockage and
biophysical properties of sodium channels Nav 1.8 and
1.9.”
Advisor: Prof. Dr. Carlos Alberto Manssour Fraga
LASSBio/UFRJ
71) Giuliana Bertozi Francisco CV-Lattes
CNPq Technical
Support Scholarship – AT NM
Time:
September 2010 to December 2011
Project: “Semicarbazone Benzaldehyde
(BS)”
Advisor: Prof. Dr. Fernando de Queiroz Cunha
Faculty of
Medicine of Ribeirao Preto
STATISTICS ACCORDING TO TYPE OF
SCHOLARSHIP
CNPq
JUNIOR POST-DOCTORATE (PDJ) –
16
INDUSTRIAL TECHNOLOGICAL
DEVELOPMENTL (DTI-1) – 9
INDUSTRIAL TECHNOLOGICAL
DEVELOPMENT (DTI-2) – 4
INDUSTRIAL TECHNOLOGICAL
DEVELOPMENT (DTI-3) – 12
INDUSTRIAL AND TECHNOLOGICAL
INITIATION (ITI) - 4
MID-LEVEL TECHNICAL SUPPORT
(ATNM) – 12
HIGHER LEVEL TECHNICAL SUPPORT
(ATNS) - 6
SCIENTIFIC INITIATION (IC) -
10
Total CNPq Scholarships– 73
CAPES
MASTER DEGREE - 3
DOCTORATE - 4
PARTIAL FOREIGN EXCHANGE
DOCTORATE - 2
POST-DOCTORATE ABROAD – 1
Total CAPES Scholarships–
10
INCT-INOFAR Researchers
Coordinator:
-
Eliezer J. Barreiro (UFRJ) CV-Lattes
Vice-Coordinator:
-
Fernando de Queiroz Cunha (USP-RP) CV-Lattes
Associated Laboratory Supervisors:
-
Adelaide Maria de Souza Antunes (UFRJ) CV-Lattes
-
Angelo da Cunha Pinto (UFRJ) CV-Lattes
-
Carlos Alberto Manssour Fraga (UFRJ) CV-Lattes
-
Carlos Alberto Tagliati (UFMG) CV-Lattes
-
Carlos Mauricio Rabello de Sant'Anna (UFRRJ)
CV-Lattes
-
Claudia do O Pessoa CV Lattes
-
Claudio Viegas
Junior (UNIFAL) CV-Lattes
-
Elizabeth Igne Ferreira (USP-SP) CV-Lattes
-
Francisco Jose Roma Paumgartten (FIOCRUZ/RJ - ENSP)
CV-Lattes
-
Francisco Radler de Aquino Neto (UFRJ) CV-Lattes
-
François Germain Noel (UFRJ) CV-Lattes
-
Gisele Zapata-Sudo (UFRJ) CV-Lattes
-
Heloisa de Oliveira Beraldo (UFMG) CV-Lattes
-
Laurent Emmanuel Dardenne (LNCC) CV-Lattes
-
Lidia Moreira Lima (UFRJ) CV-Lattes
-
Luiz Carlos Dias (UNICAMP) CV-Lattes
-
Magna Suzana Alexandre Moreira (UFAL) CV-Lattes
-
Manoel Odorico de Moraes Filho (UFC) CV-Lattes
-
Marcia Paranho Veloso (UNIFAL) CV-Lattes
-
Marco Aurelio Martins (FIOCRUZ/RJ) CV-Lattes
-
Margareth de Fatima Formiga Melo Diniz (UFPB)
CV-Lattes
-
Patricia Dias Fernandes (UFRJ) CV-Lattes
-
Patricia Rieken Macedo Rocco (UFRJ) CV-Lattes
-
Ricardo
Menegatti (UFG) CV-Lattes
-
Roberto Takashi Sudo (UFRJ) CV-Lattes
-
Ronaldo de Albuquerque Ribeiro (UFC) CV-Lattes
-
Sandra Elisa Haas (UNIPAMPA) – CV-Lattes
-
Stela Maris
Kuze Rates (UFRGS) CV-Lattes
-
Thereza Christina Barja Fidalgo (UERJ) CV-Lattes
-
Valeria de Oliveira (UFG) CV-Lattes
Researchers
-
Adriana Ribeiro Silva (FIOCRUZ/RJ) CV-Lattes
-
Araken dos Santos Werneck Rodrigues (LNCC) –
CV-Lattes
-
Antonio Carlos Doriguetto (UNIFAL) CV-Lattes
-
Barbara Vasconcellos da Silva (UFRJ) CV-Lattes
-
Camila Silva de Magalhaes (UFRRJ) CV-Lattes
-
Carolina Horta Andrade (UFG) CV-Lattes
-
Catarina de Nigris Del Cistia (UFRRJ) CV-Lattes
-
Claudia Moraes de Rezende (UFRJ) CV-Lattes
-
Cristina Setim Freitas (USP/RP) CV-Lattes
-
Cristiane Sousa Nascimento Baez Garcia (UFRJ)
CV-Lattes
-
Debora Gonçalves Xisto (UFRJ) CV-Lattes
-
Edna Alves dos Anjos-Valotta (FIOCRUZ/RJ) CV-Lattes
-
Eliane Aparecida Campesatto Mella (UFAL) CV-Lattes
-
Fernanda
Antunes (UENFE) CV-Lattes
-
Helio de Mattos Alves (UFRJ) CV-Lattes
-
Helio Jose Correa Barbosa (LNCC) CV-Lattes
-
Henrique Marcelo Gualberto (UFRJ) CV-Lattes
-
Ian Castro-Gamboa (UNIFAL) CV-Lattes
-
Joao Batista Neves da Costa (UFRRJ) CV-Lattes
-
Jose Ricardo Sabino (UFG) CV-Lattes
-
Kennia Rocha Rezende (UFG) CV-Lattes
-
Leticia Veras Costa-Lotufo (UFC) CV-Lattes
-
Luciano Morais Liao (UFG) CV-Lattes
-
Magda Fraguas Serra (FIOCRUZ/RJ) CV-Lattes
-
Marcelo Henrique dos Santos (UNIFAL) CV-Lattes
-
Marcio de Matos Coelho (UFMG) CV-Lattes
-
Margarete Manhaes Trachez (UFRJ) CV-Lattes
-
Maria Regina Gomes Carneiro (FIOCRUZ/RJ) CV-Lattes
-
Mariana Lima Vale (UFC) CV-Lattes
-
Marize Campos Valadares Bozinis (UFG) CV-Lattes
-
Matheus Lavorenti Rocha (UFG) CV-Lattes
-
Nelilma Correia Romeiro (UFRJ) CV-Lattes
-
Newton
Goncalves de Castro (UFRJ) CV-Lattes
-
Patricia Machado Rodrigues e Silva Martins
(FIOCRUZ/RJ) CV-Lattes
-
Pedro Leme Silva (UFRJ) CV-Lattes
-
Priscila Vanessa Zabala Capriles Golliat (LNCC)
CV-Lattes
-
Priscilla Christina Olsen (FIOCRUZ/RJ) CV-Lattes
-
Raquel Carvalho Montenegro (UFC) CV-Lattes
-
Renato Sergio Balao Cordeiro (FIOCRUZ/RJ) –
CV-Lattes
-
Rosangela de Oliveira Alves Carvalho (UFG) CV-Lattes
-
Sharlene Lopes Pereira (UFRJ) CV-Lattes
-
Tatiana Paula Teixeira Ferreira (FIOCRUZ/RJ)
CV-Lattes
-
Thaiana da Cunha Ferreira Mendes (UFRJ) CV-Lattes
-
Thiago Mattar Cunha (USP/RP) CV-Lattes
-
Ulisses Gazos Lopes (UFRJ) CV-Lattes
-
Vinicius de Frias Carvalho (FIOCRUZ/RJ) CV-Lattes
-
Virginia Veronica de Lima (UFRJ) CV-Lattes
Scholars
-
Adriano Siqueira Vieira (UNICAMP) CV-Lattes
-
Allan Kardec Nogueira de Alencar (UFRJ) CV-Lattes
-
Alan Rodrigues de Sousa (UFRJ) CV-Lattes
-
Alexandra Basilio Lopes (UFRJ) CV-Lattes
-
Ana Carla Dos Santos (UFRJ) CV-Lattes
-
Ana Cristina da Mata Silva (UFRJ) CV-Lattes
-
Ana Gabriela de
Almeida Silva
-
Ana Katia dos Santos (UFRJ) CV-Lattes
-
Ana Maria Calçado dos Santos (UFG) CV-Lattes
-
Andre Victor Pereira (UNIFAL) CV-Lattes
-
Arthur Eugen
Kümmerle (UFRJ) CV-Lattes
-
Arthur Henrique Freitas do Prado (UFRJ) CV-Lattes
-
Barbara Assis Novak (UFRJ) CV-Lattes
-
Bianca Torres Ciabarella (FIOCRUZ/RJ) CV-Lattes
-
Bruno Coelho Cavalcanti (UFC) CV-Lattes
-
Carlos Eduardo da Silva Monteiro (UFRJ) CV-Lattes
-
Carolina
Maldonado Galassi CV-Lattes
-
Carolina Neris Cardoso (UFMG) CV-Lattes
-
Clemilson
Berto Junior (UFRJ) CV-Lattes
-
Daniel Nascimento do Amaral (UFRJ) CV-Lattes
-
Danilo Roman
Campos CV-Lattes
-
Douglas Rodrigues Outeiro de Oliveira (UFRJ)
CV-Lattes
-
Edna Maria de Oliveira Ferreira CV-Lattes
-
Elsa Moreno de Viguri (UNICAMP) CV-Lattes
-
Fabricio Maia da Silva Salvador (UFRJ) CV-Lattes
-
Fanny
Nascimento Costa CV-Lattes
-
Gabrielle Luck de Araujo (UFMG) CV Lattes
-
Geovana Barbara Ferreira Mendes (UFG) CV-Lattes
-
Givanildo Santos da Silva (UFRJ) CV-Lattes
-
Giuliana Bertozi Francisco (USP-RP) CV-Lattes
-
Hannah Carolina T. Domingos (UFRJ) CV-Lattes
-
Isabella Pires
Ferreira CV
Lattes
-
Javier Ceras Aresse (UNICAMP) CV-Lattes
-
Jean Marcell Marcelino Pena (UFRJ) CV-Lattes
-
Jessica Silva dos Santos (UFRJ) CV-Lattes
-
Juliana Fátima Vilachã Madeira Rodrigues dos Santos
(UFRJ) CV Lattes
-
Juliane Cabral
Silva CV-Lattes
-
Julio Beltrame Daleprane (FIOCRUZ/RJ) CV-Lattes
-
Leandro Louback da Silva (UFRJ) CV-Lattes
-
Leila de Souza Conegero (UNICAMP) CV-Lattes
-
Leonardo Ferreira de Oliveira CV-Lattes
-
Lethycia
Machado Tannuri CV-Lattes
-
Lidilhone
Hamerski Carbonezi (UFRJ) CV-Lattes
-
Lucia Beatriz Torres (UFRJ) CV-Lattes
-
Luciana Almeida Piovesan (UFRJ) CV-Lattes
-
Luciano da Silva Santos (UFRJ) CV-Lattes
-
Luis Eduardo Reina Gamba CV-Lattes
-
Maitia Labora
Poggi CV-Lattes
-
Manuella de Lima Toccafondo Vieira CV Lattes
-
Maria de Fatima do Nascimento Alfredo (UFRJ)
CV-Lattes
-
Mariana Trad R. da Motta (UFRJ) CV-Lattes
-
Marlon Daniel Lima Tonin (UFRJ) CV-Lattes
-
Marcus Vinicius dos Santos (UFMG) CV-Lattes
-
Moacir
Kaiser CV-Lattes
-
Nailton Monteiro do Nascimento Junior (UFRJ)
CV-Lattes
-
Natalia Lacerda Alencar Peixoto CV-Lattes
-
Natalia Medeiros de Lima (UFRJ) CV-Lattes
-
Nathalia Freitas Emiliano (UFMG) CV-Lattes
-
Pedro Gabriel D. L. Pereira (UFRJ) CV-Lattes
-
Priscila de Paula Cabral (UFRJ) CV-Lattes
-
Raquel de Oliveira Lopes (UFRJ) CV-Lattes
-
Roberta Tesch (UFRJ) CV-Lattes
-
Rodolfo do Couto Maia (UFRJ) CV-Lattes
-
Sabrina Teixeira Martinez (UFRJ) CV-Lattes
-
Samira de Sa e Souza (UFMG) CV-Lattes
-
Sarah da Silva Nunes (UFG) CV-Lattes
-
Tais Rubia dos Santos (UFRJ) CV-Lattes
-
Thais Emmanoelle Tavares Pompeu CV-Lattes
-
Thiago Stevanatto Sampaio (UFRJ) CV-Lattes
-
Vinicius Frias de Carvalho (FIOCRUZ/RJ) CV-Lattes
-
Wallace Carvalho Ferreira (UFMG) CV-Lattes